Copyright
©The Author(s) 2021.
World J Stem Cells. Jun 26, 2021; 13(6): 568-593
Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.568
Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.568
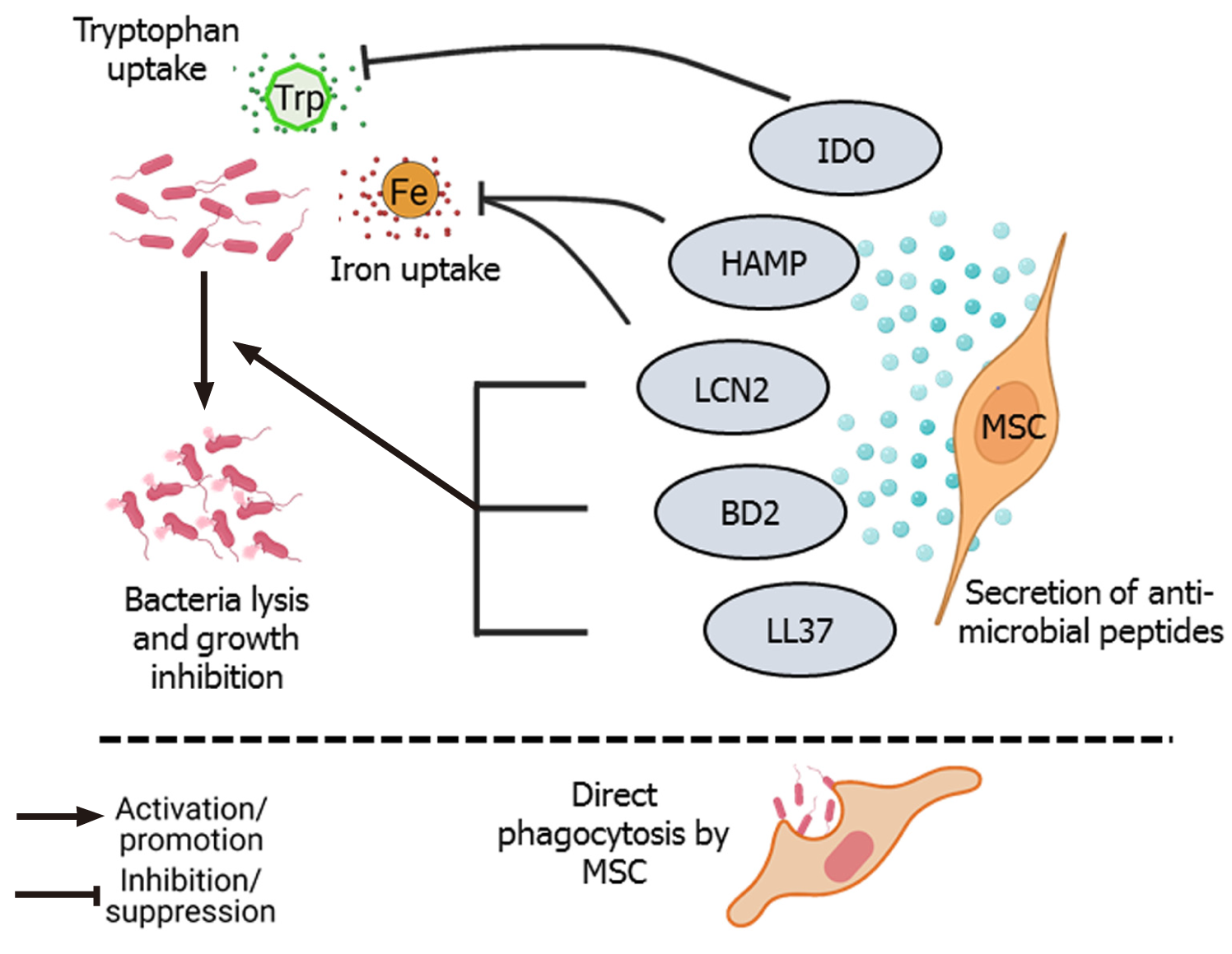
Figure 1 Direct bacterial lysis and phagocytosis.
Secretion of antimicrobial peptides such as LL-37, β-defensin 2 (BD2), lipocalin 2 (LCN2), hepcidin antimicrobial peptide (HAMP), and indoleamine 2, 3-dioxygenase (IDO) by mesenchymal stem cells (MSCs) have bactericidal effects[23,24,30,31]. LCN2 and HAMP inhibit iron (Fe) uptake by bacterial cells, and IDO inhibits the uptake of the essential amino acid tryptophan (Trp), leading to growth inhibition and death of bacterial cells[27,29]. MSCs can also directly phagocytose bacteria[32].
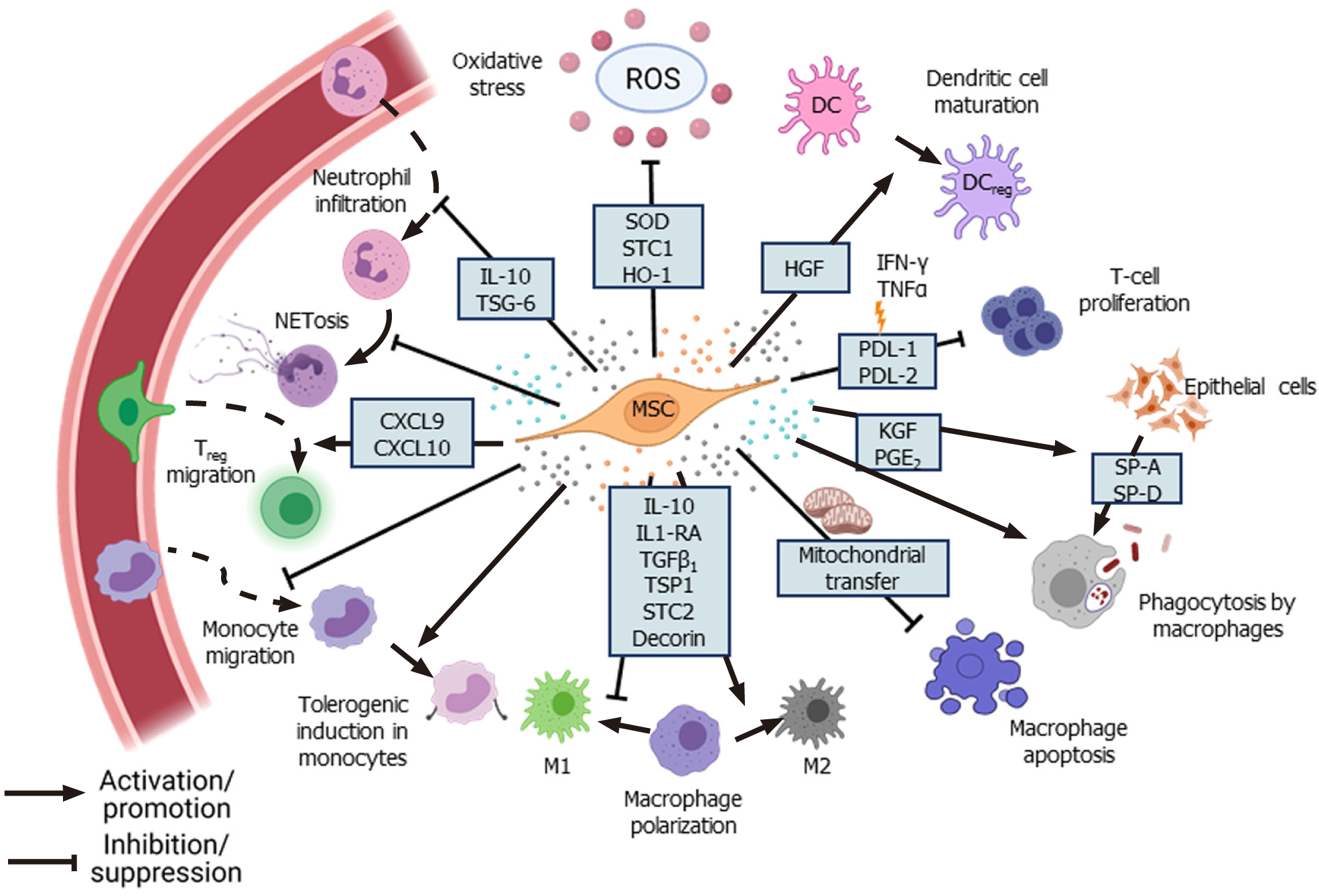
Figure 2 Immunomodulation by mesenchymal stem cells.
Mesenchymal stem cells (MSCs) secrete various paracrine factors and chemokines to reduce inflammation at the site of injury by inhibiting infiltration of neutrophils, pro-inflammatory monocytes, and maturation of dendritic cells[39,62,219]. MSCs promote anti-inflammatory M2 phenotype in macrophages, improve phagocytic ability and increase their survival via mitochondrial transfer[12,43-45,48]. MSCs under inflammatory conditions inhibit T-cell proliferation via secretion of programmed death-ligand 1 (PD-L1) and PD-L2[54]. MSCs induce a “tolerogenic” phenotype in monocytes and promote migration of regulatory T cells to the site of infection[58,60]. Ang1: Angiopoietin-1; EVs: Extracellular vesicles; HGF: Hepatocyte growth factor; KGF: Keratinocyte growth factor; LXA: LipoxinA4; MMPs: Matrix metalloproteinases; VEGF: Vascular endothelial growth factor.
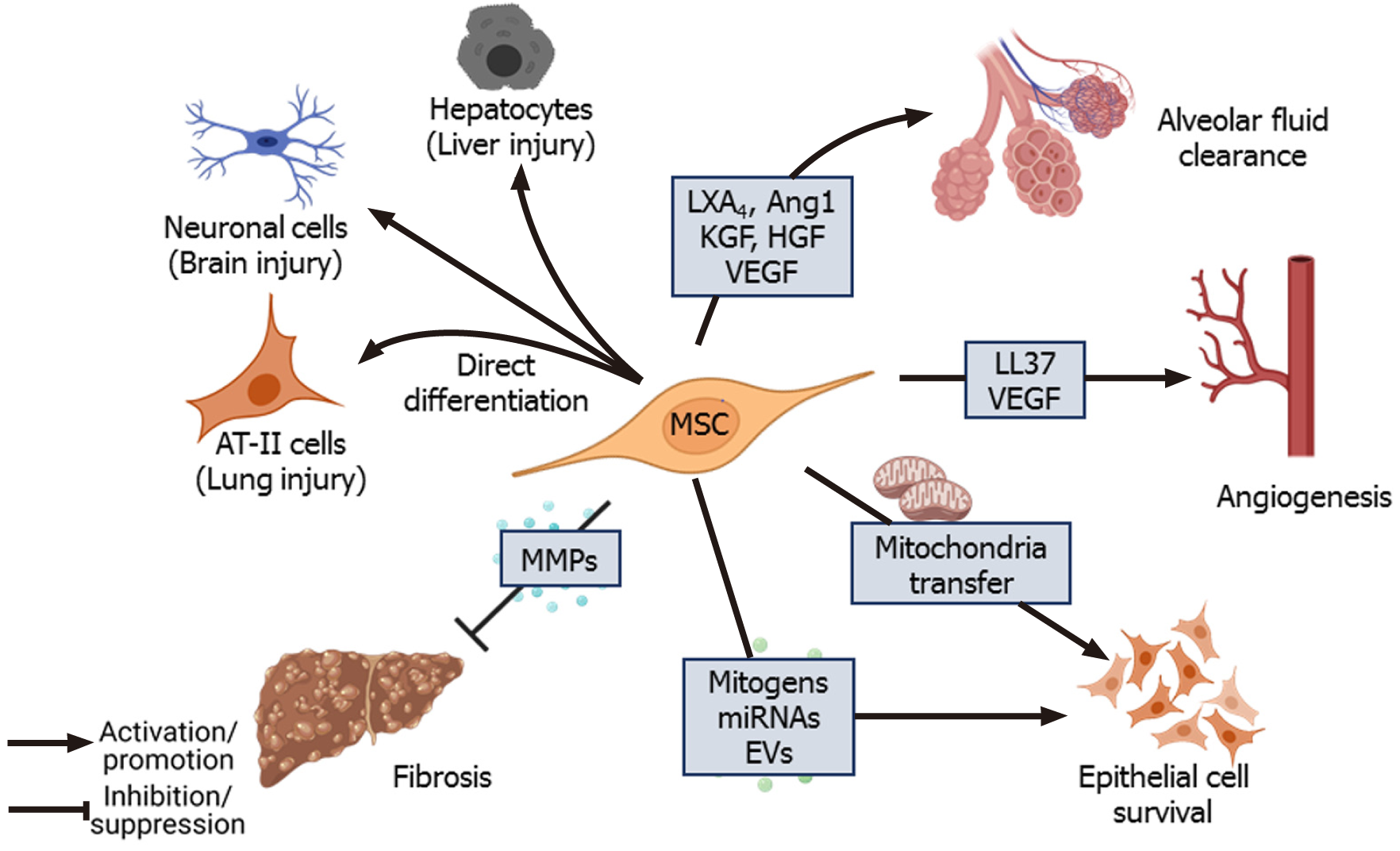
Figure 3 Tissue repair and regeneration.
Mesenchymal stem cells (MSCs) have multilineage differentiation potential and can differentiate into cells of target tissue during injury. MSCs also reduce collagen deposition and fibrosis at the site of injury by secreting matrix metalloproteinases[114,115]. Mitogens secreted directly or packaged in extracellular vesicles secreted by MSCs, mitochondrial transfer from MSCs to the injured cells promote proliferation and survival of lung epithelial cells[87,88]. MSCs also secrete pro-angiogenic factors such as vascular endothelial growth factor[108] and LL-37 to promote angiogenesis[25]. In the context of lung injury, lipoxinA4, angiopoietin-1, keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor promote alveolar fluid clearance, which improves gas exchange in the lungs[68,70,94,95]. CXCL: C-X-C motif ligand; DC: Dendritic cell; HGF: Human growth factor; HO-1: Heme oxgenase-1; IFNγ: Interferon γ; IL-10: Interleukin 10; IL1RA: Interleukin 1 receptor antagonist; PD-L1: Programmed death-ligand 1; PGE: Prostaglandin E2; ROS: Reactive oxygen species; SOD: Superoxide dismutase; SP: Surfactant protein; STC: Stanniocalcin; TSG-6: Tumor necrosis factor α-stimulated gene-6; TNFα: Tumor necrosis factor α; TGF: Transforming growth factor; Treg: regulatory T cell; TSP: Thrombospondin.
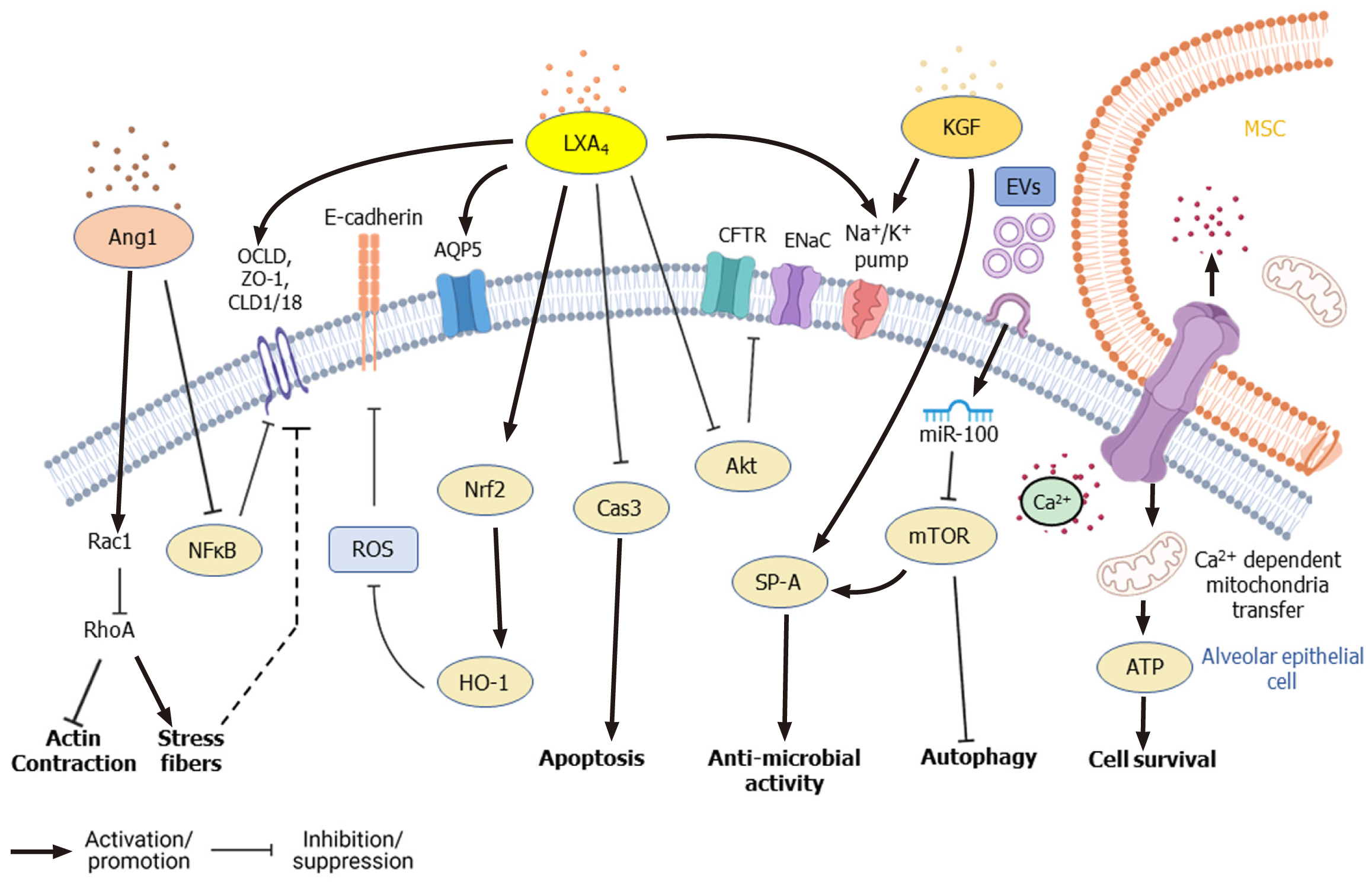
Figure 4 Signaling pathways modified by mesenchymal stem cells in alveolar lung epithelial cells during lung injury.
Mesenchymal stem cells (MSCs) reduce alveolar permeability and promote survival of alveolar epithelial cells through secretion of lipoxinA4 (LXA4), angiopoietin (Ang1), keratinocyte growth factor (KGF), along with extracellular vesicles (EVs). During acute lung injury (ALI), LXA4 inhibits apoptosis of alveolar epithelial cells by inhibiting caspase-3[104]. LXA4 also upregulates ion channels like Na+/K+ pump and rescues expression of cystic fibrosis transmembrane conductance regulator and epithelial Na+ channel by inhibiting Akt phosphorylation[96]. LXA4 also rescues E-cadherin expression by reducing reactive oxygen species via modulation of nuclear factor erythroid 2–related factor 2/heme oxygenase-1 expression[103]. LXA4, as well as Ang1, promote the expression of tight junction proteins like occludin, Zona occludens, and claudin 1[64,94,100]. Ang1 also rescues the expression of claudin 18 by inhibiting NF-κB. Ang1 upregulates Rac1, which promotes the formation of adherens and tight junctions[106]. KGF promotes the secretion of surfactant protein SP-A by alveolar epithelial cells and promotes the expression of Na+/K+ pump[87]. Inhibition of mTOR by miR-100 present in EVs secreted by MSCs increases autophagy, thereby promotes survival of epithelial cells during ALI. Ca2+ dependent transfer of mitochondria from MSCs, through gap junctions formed by Cx43, increases ATP generation in epithelial cells and improves their survival[112]. Akt: Protein kinase B; Ang1: Angiopoietin; AQP5: Aquaporin-5; ATP: Adenosine triphosphate; Cas3: Caspase-3; CFTR: Cystic fibrosis transmembrane conductance regulator; CLD1: Claudin 1; ENaC: Epithelial Na+ channel; EVs: Extracellular vesicles; HO-1: Heme oxygenase-1;KGF: Keratinocyte growth factor; LXA4: LipoxinA4; MSC: Mesenchymal stem cell; mTOR: Mammalian target of rapamycin; NF-κB: Nuclear factor-kappa B; Nrf2: Nuclear factor erythroid 2-related factor 2; OCLD: Occludin; Rac1: Rac family small GTPase 1; RhoA: Ras homolog family member A; ROS: Reactive oxygen species; ZO-1: Zona occludens.
- Citation: Sharma A, Chakraborty A, Jaganathan BG. Review of the potential of mesenchymal stem cells for the treatment of infectious diseases. World J Stem Cells 2021; 13(6): 568-593
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/568.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.568