Copyright
©The Author(s) 2020.
World J Stem Cells. Jul 26, 2020; 12(7): 604-611
Published online Jul 26, 2020. doi: 10.4252/wjsc.v12.i7.604
Published online Jul 26, 2020. doi: 10.4252/wjsc.v12.i7.604
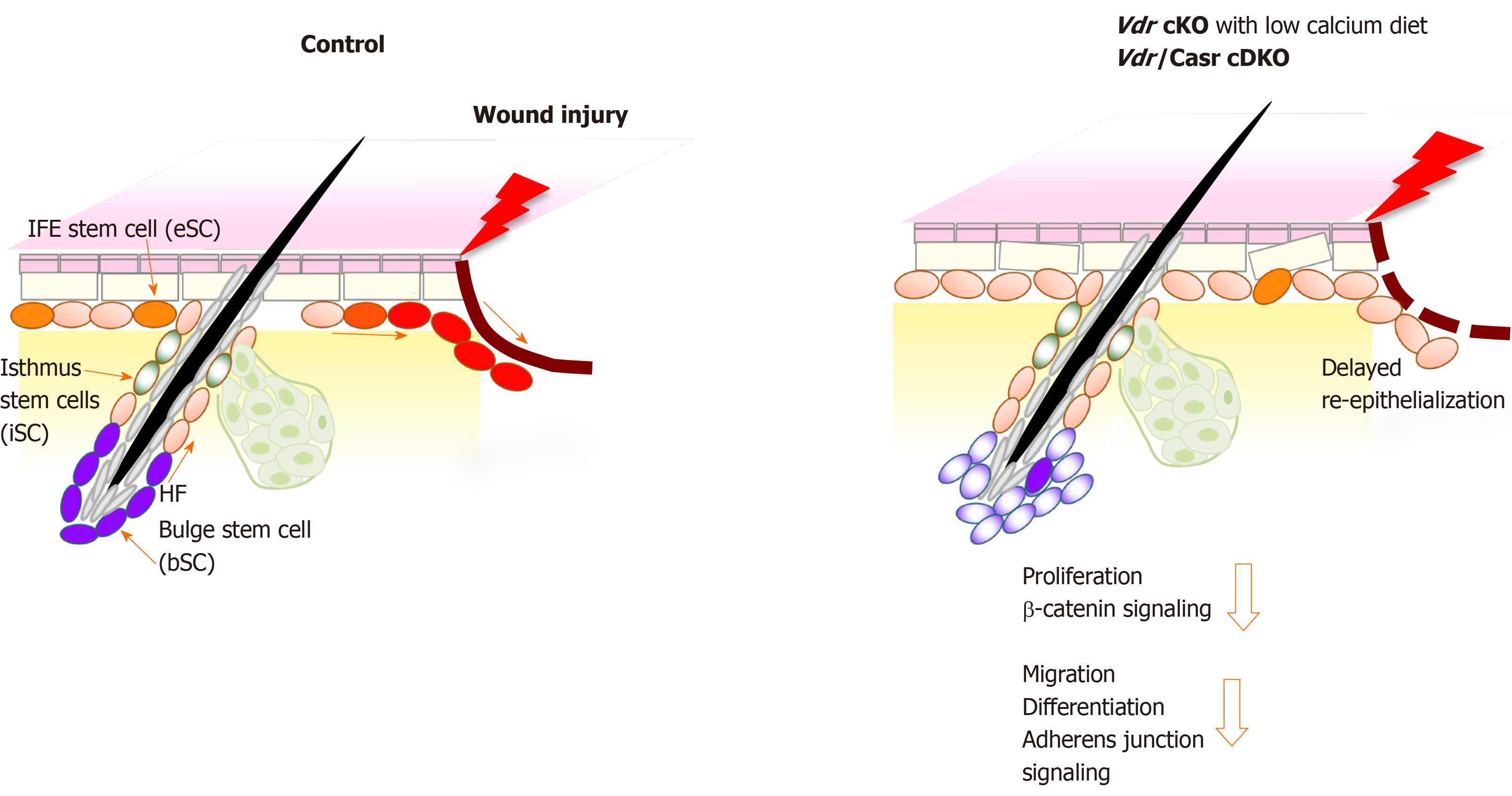
Figure 1 Schematic model showing that deficiency in both vitamin D receptor and calcium sensing receptor prevents proliferation and migration of keratinocytes thus delaying wound re-epithelialization of the wounded epidermis.
The location of the different stem cells (SC) niches in skin is shown; stem cells in the hair follicle bulge (bSC shown in purple color) required for hair cycling, isthmus stem cells (iSC, green) in the junctional zone of the upper hair follicle responsible for sebaceous gland renewal, and epidermal stem cells (eSC, orange) in the interfollicular epidermis (IFE) responsible for epidermal regeneration. Upon injury of normal skin (left panel), these stem cells at the wound edge are activated to proliferate, shifting their normal cell fate to re-populate the disrupted epidermis by migrating to the wound and differentiating to re-epithelialize the wound (red color). In contrast, vitamin D receptor (Vdr) conditional knockout (cKO) mice fed a calcium deficient diet and Vdr/Casr double KO (cDKO) mice show defects in these stem cells that reduce their responses to wounding (right panel). The number of bSC and eSC decreases in Vdr cKO demonstrating defects in their self-renewal. Both Vdr cKO and cDKO mice have decreased injury induced proliferation of these stem cells associated with a reduction in β-catenin signaling. Delayed re-epithelialization is accompanied with defects in migration and differentiation of these stem cells, mediated by decreased AJ signaling. cKO: Conditional knockout; cDKO: Conditional double knockout.
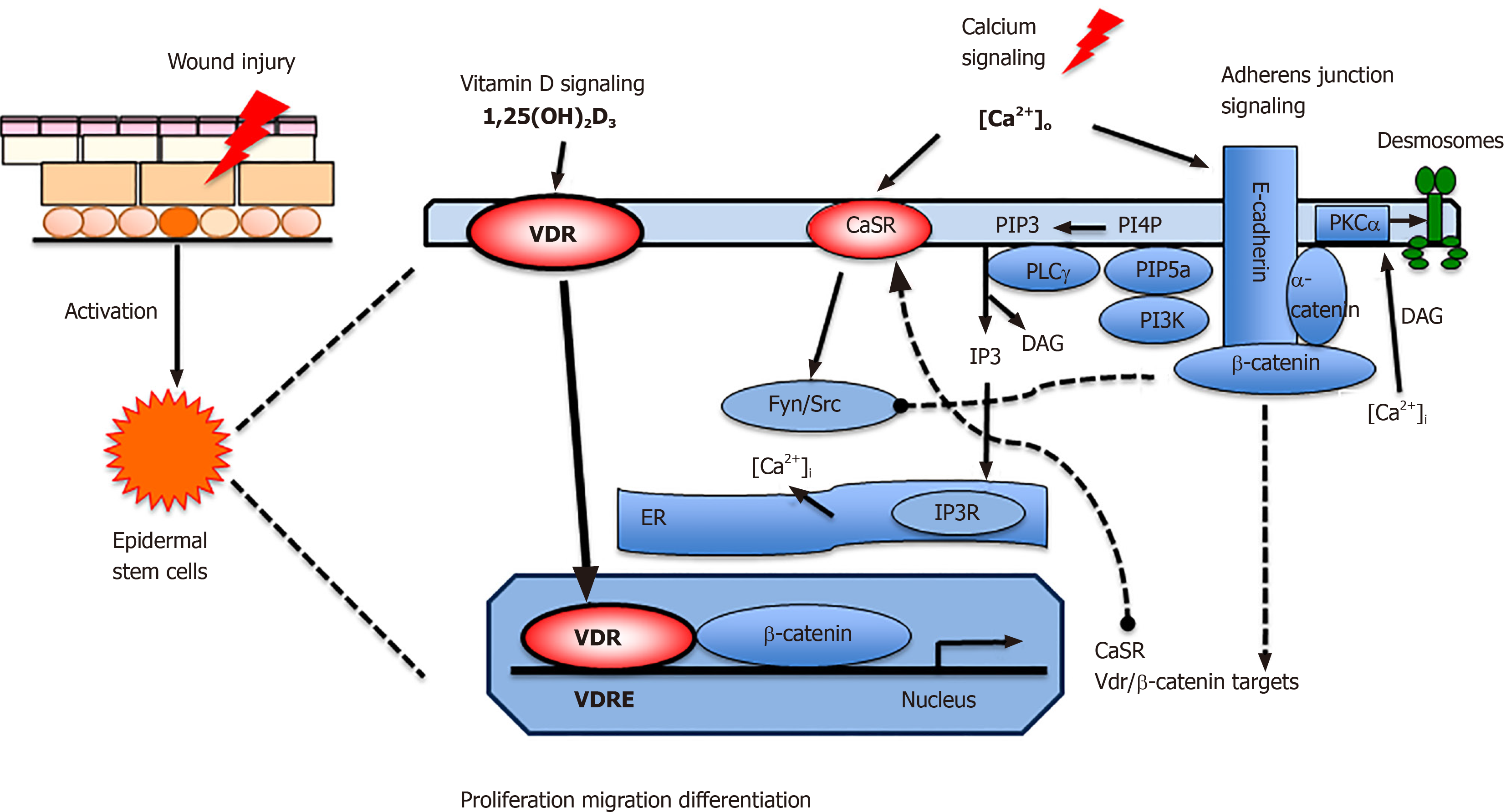
Figure 2 Proposed model in which vitamin D and calcium signaling mutually regulate β-catenin and AJ signaling essential for wound re-epithelialization.
First, vitamin D receptor (VDR) may partner with β-catenin in the nucleus to regulate the expression of β-catenin target genes such as Cyclin D1 to promote proliferation of stem cells when the skin is wounded. Subsequently extracellular calcium [Ca]o in collaboration with VDR stimulates E-cadherin/catenin complex formation to promote keratinocyte differentiation while reducing the proliferative stimulus by sequestering β-catenin in the membrane. The E-cadherin/catenin complex formation is facilitated by the activation of Fyn/Src kinases by the CaSR which phosphorylate the catenins required for their recruitment into the E-cadherin/catenin complex. The E-cadherin/catenin complex not only provides a reservoir of β-catenin in the membrane but also includes a link to the cytoskeleton via a catenin enabling cell migration and differentiation essential for epidermal remodeling during wound re-epithelialization. Moreover, enzymes within the E-cadherin/catenin complex sequentially phosphorylate PIP to PIP3, that activates PLCγ, that in turn hydrolyzes PIP2 to DAG and IP3. The latter stimulates the IP3 receptor in subcellular organelles (ER and Golgi in keratinocytes) to release calcium. DAG, on the other hand along with calcium activates PKCa, the enzyme that activates the AP-1 transcription factors involved in the expression of differentiation markers in keratinocytes as well as phosphorylation of desmoplakin, which alters the desmosomal structure facilitating migration of the keratinocytes to re-epithelialize the wounds. VDR: Vitamin D receptor; CaSR: Calcium sensing receptor; DAG: Diacylglycerol;ER: Endoplasmic reticulum; PLCγ: Phospholipase γ; IP3: Inositol trisphosphate; PIP3: Phosphatidylinositol 3,4,5-trisphosphate; PI3K: Phosphatidyl inositol 3 kinase; PIP5K1a: phosphatidyl inositol 4-phosphate 5-kinase 1α.
- Citation: Oda Y, Bikle DD. Vitamin D and calcium signaling in epidermal stem cells and their regeneration. World J Stem Cells 2020; 12(7): 604-611
- URL: https://www.wjgnet.com/1948-0210/full/v12/i7/604.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i7.604