Copyright
©The Author(s) 2020.
World J Stem Cells. Dec 26, 2020; 12(12): 1553-1575
Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1553
Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1553
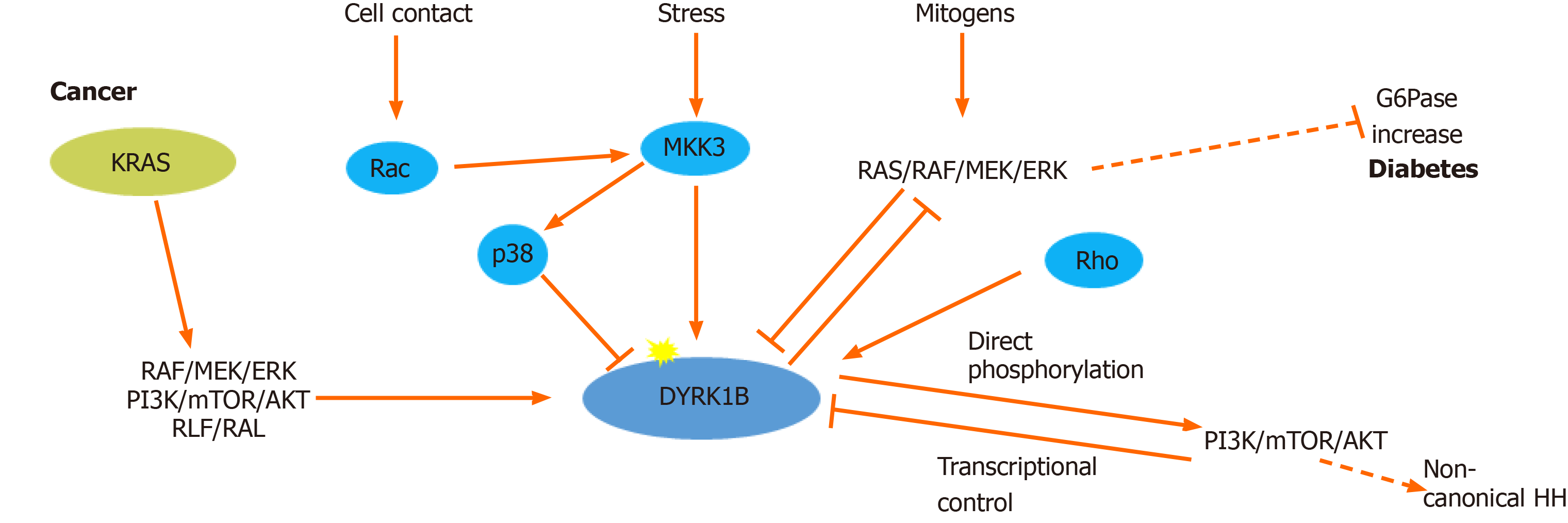
Figure 1 Regulation of dual-specificity tyrosine-regulated kinase 1B expression and activity.
Dual-specificity tyrosine-regulated kinase 1B (DYRK1B) expression and activity is regulated at transcriptional, translational and post-translational level. Rho GTPases (RhoA, Cdc42 and Rac1) promote transcriptional up-regulation of DYRK1B, while serum mitogens down-regulate DYRK1B through RAS/RAF/MEK/extracellular signal-regulated kinases (ERK) signaling pathway. Under stress conditions, MKK3 activates DYRK1B and p38, while DYRK1B is physically sequestered and inhibited by p38. In cancer, DYRK1B is involved in a complex crosstalk with hedgehog (HH). Oncogenic mutant RAS (KRAS) initiates the non-canonical HH pathway through the activation of DYRK1B, via an unknown mechanism, employing several RAS effectors, such as: RAF/MEK/ERK, PI3K/AKT and RLF/RAL. DYRK1B enhances non-canonical HH signaling by promoting PI3K/mTOR/AKT signaling. Conversely, activated AKT directly inhibits expression of DYRK1B. In metabolic syndrome, which is accompanied by diabetes, DYRK1B is implicated in glucose homeostasis, promoting the expression of the key gluconeogenic enzyme glucose-6-phosphatase (G6pase), through inhibition of the RAS–RAF–MEK pathway. Dashed lines represent indirect mechanisms and yellow stars represent phosphorylations. ERK: Extracellular signal-regulated kinases.
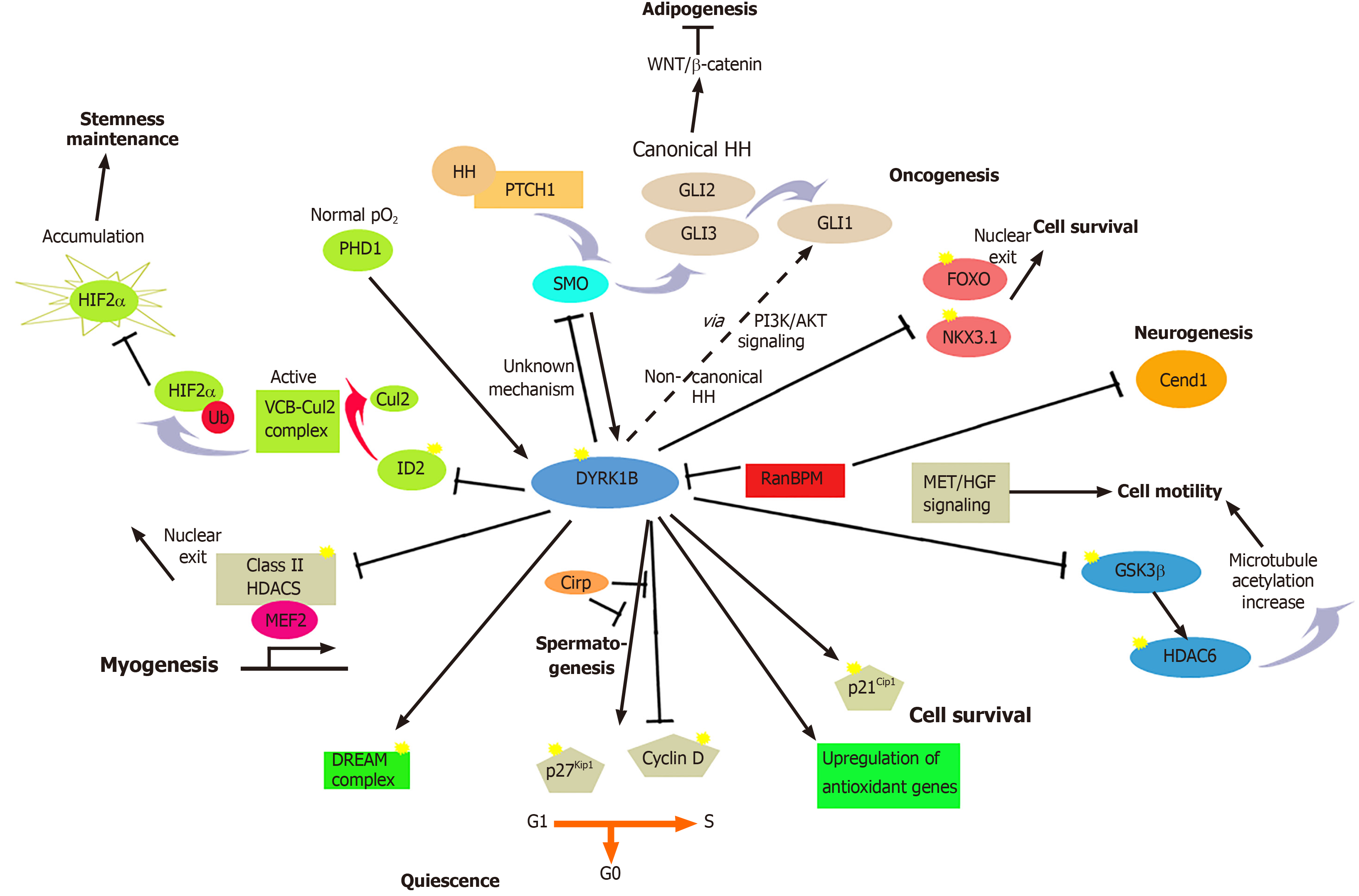
Figure 2 Summary of the major known functions of dual-specificity tyrosine-regulated kinase 1B in development and disease.
Dual-specificity tyrosine-regulated kinase 1B (DYRK1B) plays a critical role in many biological processes in development and human diseases, regulating cell cycle progression/exit, differentiation, transcription, and cell survival and motility. DYRK1B facilitates growth arrest and promotes quiescence (G0) by stabilizing cyclin-dependent kinase inhibitor p27Kip1 and destabilizing cyclin D1, via phosphorylation. The anti-proliferative function of DYRK1B occurs in myogenesis, spermatogenesis, neurogenesis, and cancer. Moreover, DYRK1B maintains quiescence by stabilization of the DREAM complex via phosphorylation of LIN52, a subunit of MuvB in the complex. In oncogenesis, DYRK1B induces degradation of the tumor suppressor NKX3.1, while reducing the activity of tumor suppressors and apoptotic promoters of the forkhead box O (FOXO) family, resulting in survival of cancer cells and enhancement of oncogenic GLI1 activity. The prosurvival function of DYRK1B in myogenesis and cancer is mediated through phosphorylation of p21Cip1, DYRK1B counteracts oxidative stress by reducing intracellular levels of oxygen reactive species (ROS) through the up-regulation of antioxidant genes. DYRK1B modulates stemness of cancer stem cells. In normoxia, oxygen-sensing prolyl-hydroxylase (PHD1) activates DYRK1B, which inactivates ID2, makes it unable to displace the Cul2 component from the VCB-Cul2 ubiquitin ligase complex, which remains active, promoting HIF2α degradation. In hypoxia, PHD1 and DYRK1B are inactivated, leading to activated ID2 and resulting in HIF2α accumulation that facilitates cancer stem cell maintenance. DYRK1B is involved in a complex cross-talk with hedgehog (HH). DYRK1B inhibits canonical HH signaling initiated by Smoothened (SMO), while it promotes non-canonical HH signaling by promoting PI3K-AKT-mediated stability of the GLI1 transcription factor. In metabolic syndrome, DYRK1B inhibits sonic hedgehog (SHH) and Wnt signaling, enhancing adipogenesis. In spermatogenesis, DYRK1B interacts with cold-inducible RNA-binding protein (Cirp), resulting in destabilization of p27Kip1 and cyclin D1 stabilization that promote cell cycle progression of undifferentiated spermatogonia. In myogenesis, DYRK1B inactivates Class II histone deacetylases (HDACs), resulting in myogenic regulatory factor (MEF) 2-dependent transcription of myogenic genes. In neurogenesis, tripartite functional interactions between DYRK1B, RanBPM and the neuronal protein Cend1 regulate the balance between cellular proliferation and differentiation. Increased levels of DYRK1B block cell motility through interaction with the adaptor protein RanBPM and Met/HGF signaling. DYRK1B modifies indirectly the microtubules, through phosphorylation of GSK3β and subsequent inactivation of HDAC6, leading to increase of microtubules acetylation. Dashed lines represent indirect mechanisms and yellow stars represent phosphorylations. ERK: Extracellular signal-regulated kinases.
- Citation: Kokkorakis N, Gaitanou M. Minibrain-related kinase/dual-specificity tyrosine-regulated kinase 1B implication in stem/cancer stem cells biology. World J Stem Cells 2020; 12(12): 1553-1575
- URL: https://www.wjgnet.com/1948-0210/full/v12/i12/1553.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i12.1553