Copyright
©The Author(s) 2020.
World J Stem Cells. Jan 26, 2020; 12(1): 70-86
Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.70
Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.70
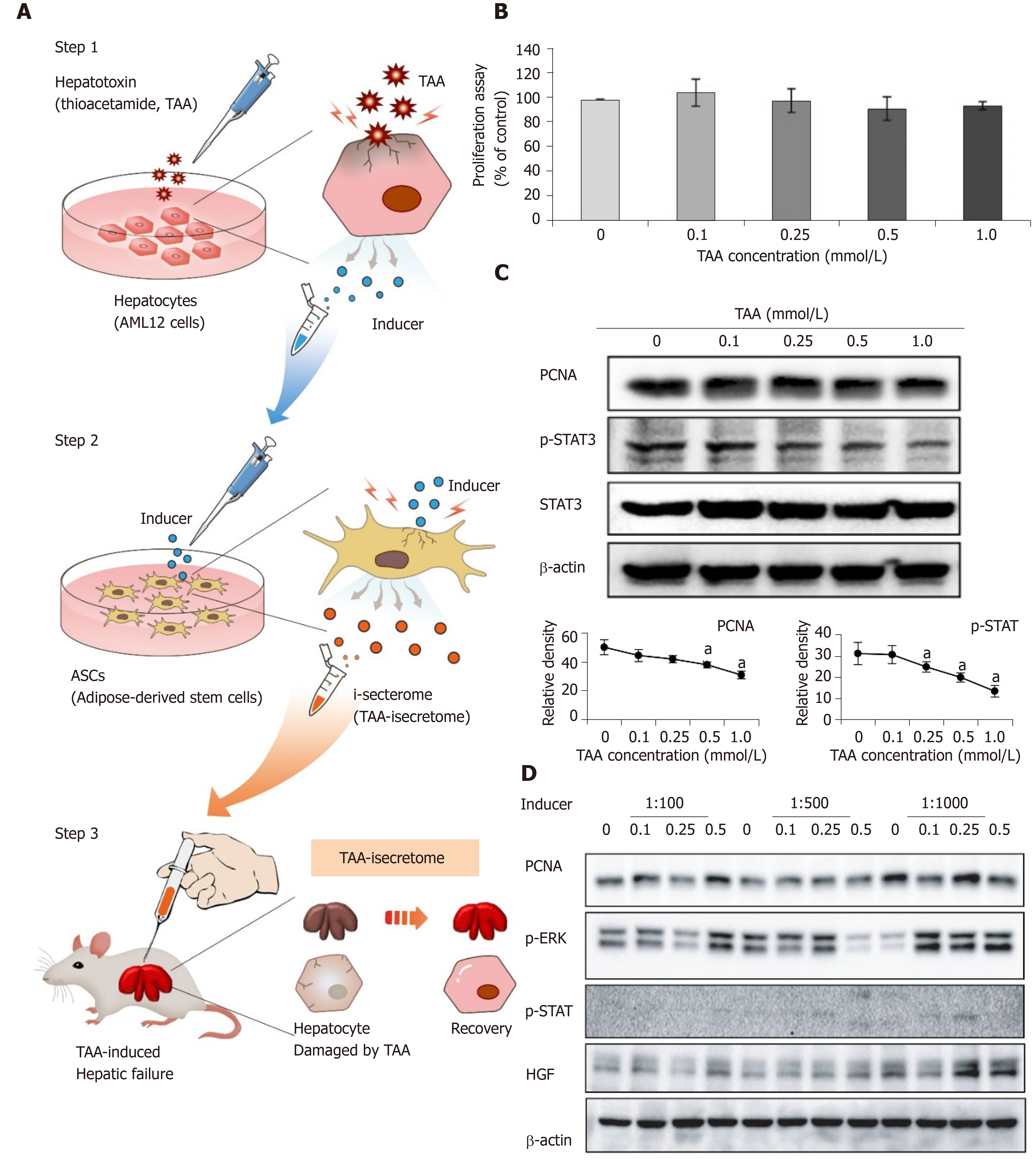
Figure 1 Generation of thioacetamide-induced secretome specialized for thioacetamide-induced toxic hepatic failure.
A: Basic concept of induced secretome (isecretome) conditioned media-based therapy. Induced secretome conditioned media therapy utilizes the mesenchymal stem cell ability to generate disease-protecting materials for specific pathogens. After identifying disease-causing materials (TAA), mouse hepatocytes were treated with TAA, and then secretary proteins (inducers) were collected. The inducers were utilized to induce adipose-derived stem cells to release the TAA-induced secretome; B: AML12 cell proliferation assay according to TAA concentrations. No significant changes in the range of tested concentrations were found; C: Western blotting showing the expression of proliferation markers in AML12 cells according to TAA concentrations. We determined that 0.25 mmol/L TAA was an “inducer” of adipose-derived stem cells because it moderately increased proliferation marker expression without reducing proliferation; D: Western blotting to determine dilution rate of inducers that maximized the expression of markers related to liver regeneration in adipose-derived stem cells. A dilution rate of 1000-fold maximally induced marker expression. Values are presented as mean ± SD of three independent experiments. aP < 0.05. PCNA: Proliferating cell nuclear antigen; p-ERK: Phosphorylated extracellular signal-regulated kinases; p-STAT3: Phosphorylated signal transducer and activator of transcription 3; TAA: Thioacetamide; ASCs: Adipose-derived stem cells.
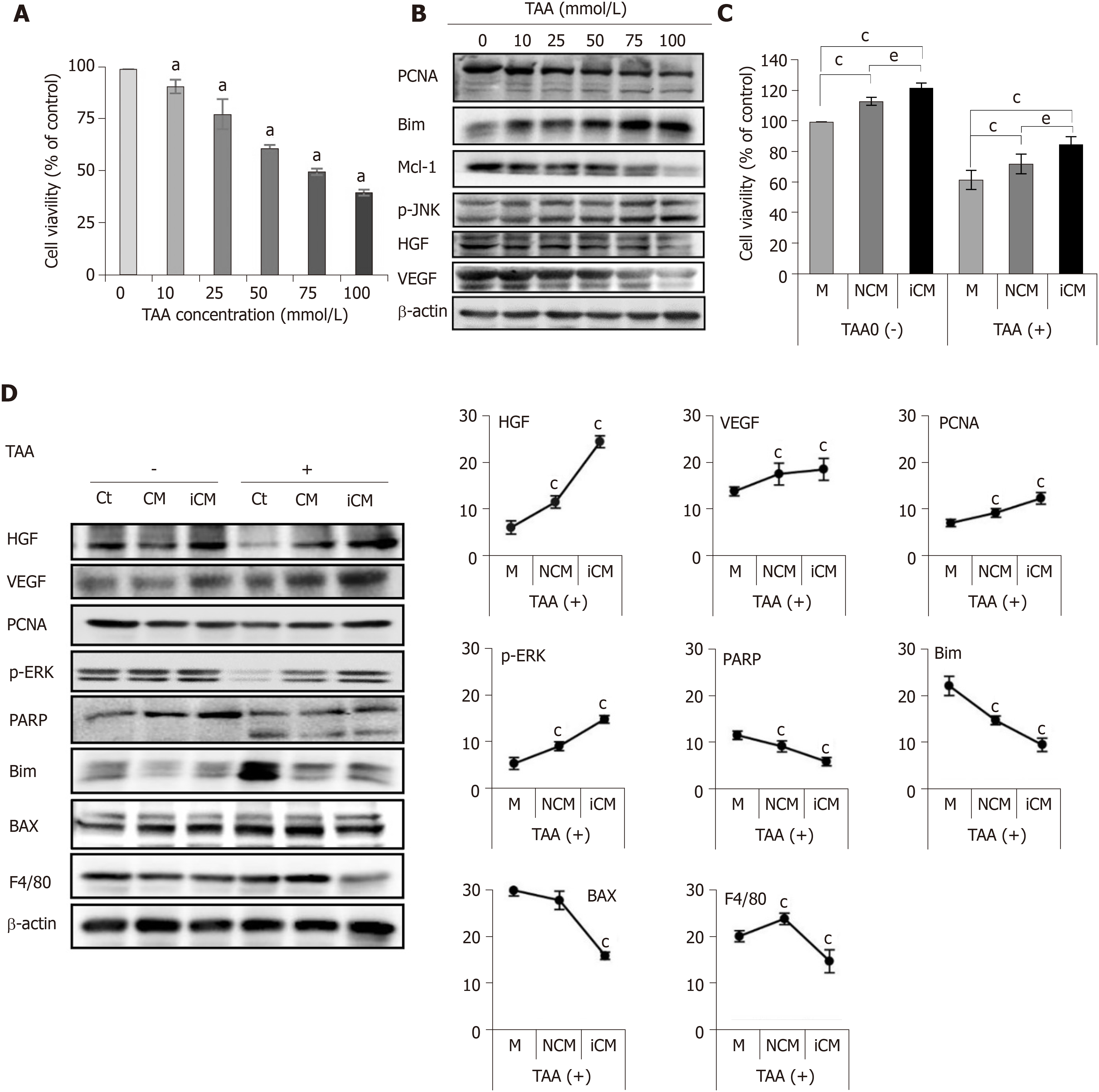
Figure 2 Determination of in vitro effects of thioacetamide-induced secretome.
A, B: Cell proliferation test (A) and western blotting (B) of AML12 cells to determine TAA concentration for generating an in vitro model of TAA-induced hepatic failure. A TAA concentration of 50 mM was used for TAA-induced hepatocytes; C, D: Effects of the TAA-induced secretome on the in vitro model of TAA-induced hepatic failure. Cell proliferation test (C) demonstrated that induced conditioned media-treated hepatocytes showed the highest cell proliferation in both normal and TAA-treated hepatocytes. In western blot analysis (D), induced conditioned media-treated hepatocytes showed the highest expression of liver regeneration markers and lowest expression of apoptosis and inflammation markers. Values are presented as mean ± SD of three independent experiments. aP < 0.05; cP < 0.05 vs media; eP < 0.05 between conditioned media and induced conditioned media. Bax: Bcl-2-like protein 4; BIM: Bcl-2-like protein 11; Ct: Control; iCM: Induced secretome group of which components were the TAA-induced secretome; HGF: Hepatocyte growth factor; M: Media group of which components were commercialized conditioned media; Mcl-1: Myeloid cell leukemia 1; CM: Normal conditioned media group of which components were the naïve secretome; PARP: Poly adenosine diphosphate-ribose polymerase; PCNA: Proliferating cell nuclear antigen; p-ERK: Phosphorylated extracellular signal-regulated kinases; p-JNK: Phosphorylated Jun N-terminal kinase; TAA: Thioacetamide; VEGF: Vascular endothelial growth factor.
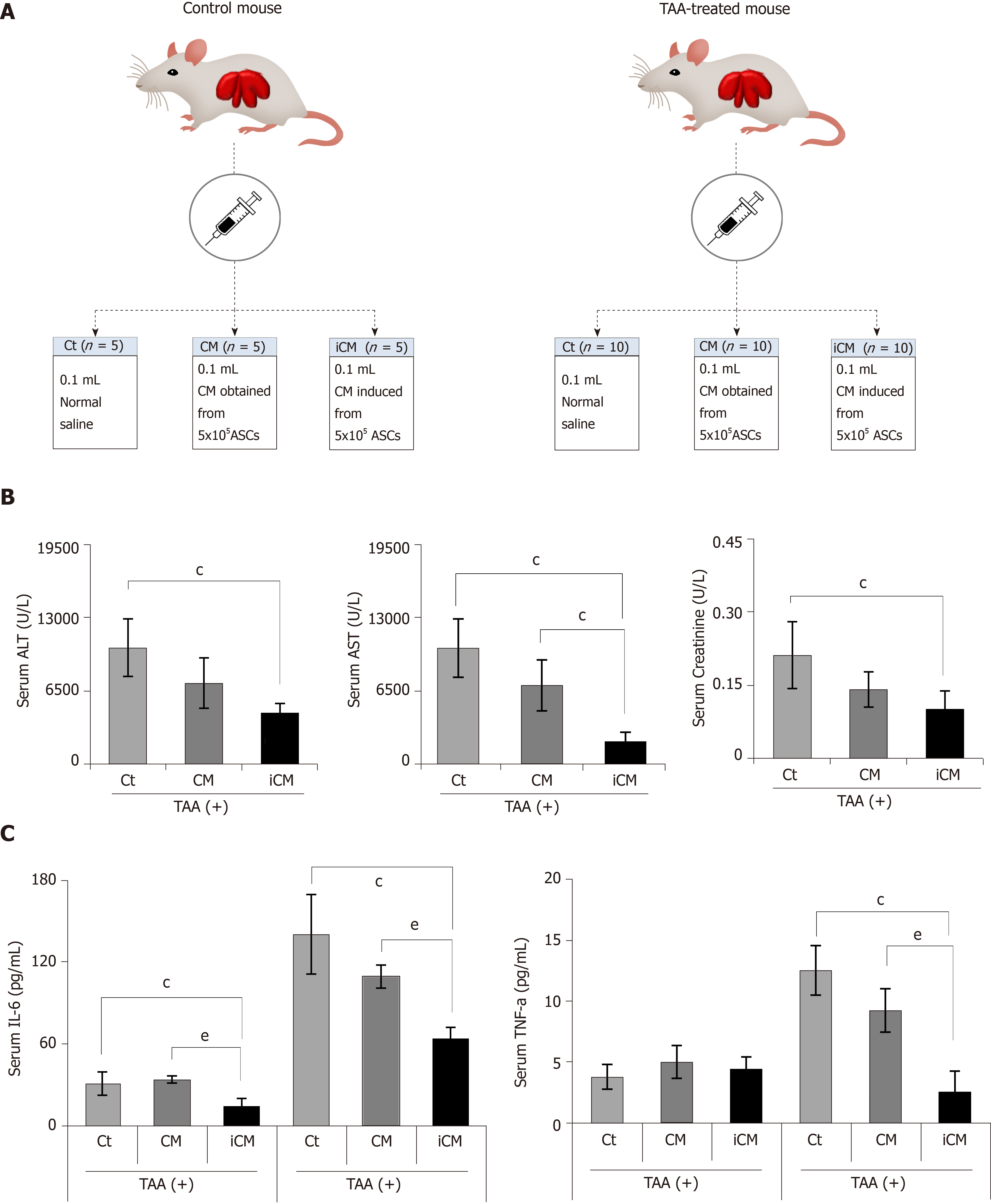
Figure 3 Animal study design and comparison of systemic effects in each group.
A: Animal study design. Control mice and TAA-treated mice were intravenously (using tail vein) infused with normal saline, conditioned media (CM), and induced CM (iCM); B: Serology tests of aspartate transaminase, alanine transaminase, and creatinine in TAA-treated mice. iCM infusion decreased the serum levels of aspartate transaminase, alanine transaminase, and creatinine to the greatest extent; C: Results of ELISA showing serum levels of inflammatory markers (IL-6 and TNF-α) in each group. iCM administration lowered the serum levels of IL-6 and TNF-α the most in TAA-treated mice. Values are presented as mean ± SD of three independent experiments. cP < 0.05 vs control (saline); eP < 0.05 between CM and iCM. ALT: Alanine transaminase; AST: Aspartate transaminase; iCM: Isecretome group of which components were the TAA-induced secretome; NCM: Normal conditioned media group of which components were the naïve secretome; PECAM: Platelet endothelial cell adhesion molecule; TAA: Thioacetamide.
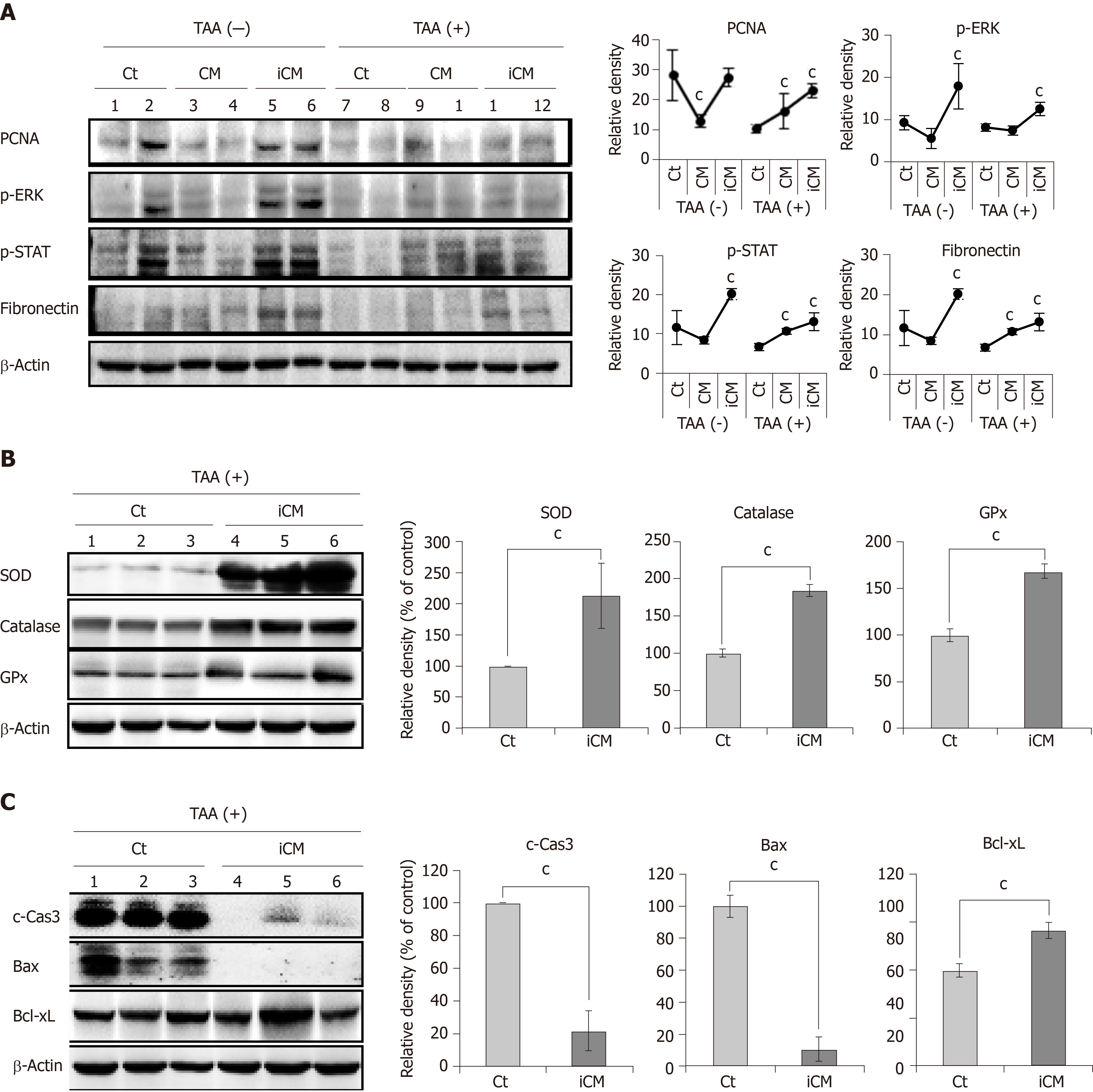
Figure 4 Molecular studies validating effects of thioacetamide-induced secretome in the mouse model of thioacetamide-induced hepatic failure.
A: Pro-proliferative actions of the TAA-induced secretome in the mouse model of TAA-induced hepatic failure. Western blot analysis comparing the expression of various markers reflecting liver regeneration and inflammation in liver specimens of each group. TAA-induced conditioned media infusion induced the highest expression of PCNA, p-ERK, p-STAT, and fibronectin in both control livers and those of TAA-treated mice; B: Antioxidant actions of TAA-induced secretome in the mouse model of TAA-induced hepatic failure. Western blot analysis was performed to compare the expression of antioxidant enzymes (SOD, catalase, and GPx) in liver specimens from each group. The TAA-induced conditioned media infusion group showed the higher expression of antioxidant enzymes in the livers of TAA-treated mice compared to in the control group; C: Anti-apoptotic actions of the TAA-induced secretome in the mouse model of TAA-induced hepatic failure. TAA-iCM infusion group showed lower expression of proapoptotic proteins (c-Caspase-3 and Bax) and higher expression of an anti-apoptotic protein (Bcl-xL) in the livers of TAA-treated mice compared to in the control group. Values are presented as mean ± SD of three independent experiments. cP < 0.05 vs control (saline). Bax: Bcl-2-like protein 4; Bcl-xL: B-cell lymphoma-extra large; c-Cas3: Cleaved caspase-3; GPx: Glutathione peroxidase; iCM: Induced secretome group of which components were the TAA-induced secretome; CM: Normal conditioned media group of which components were the naïve secretome; PCNA: Proliferating cell nuclear antigen; p-ERK: Phosphorylated extracellular signal-regulated kinases; p-STAT3: Phosphorylated signal transducer and activator of transcription 3; SOD: Superoxide dismutase; TAA: Thioacetamide.
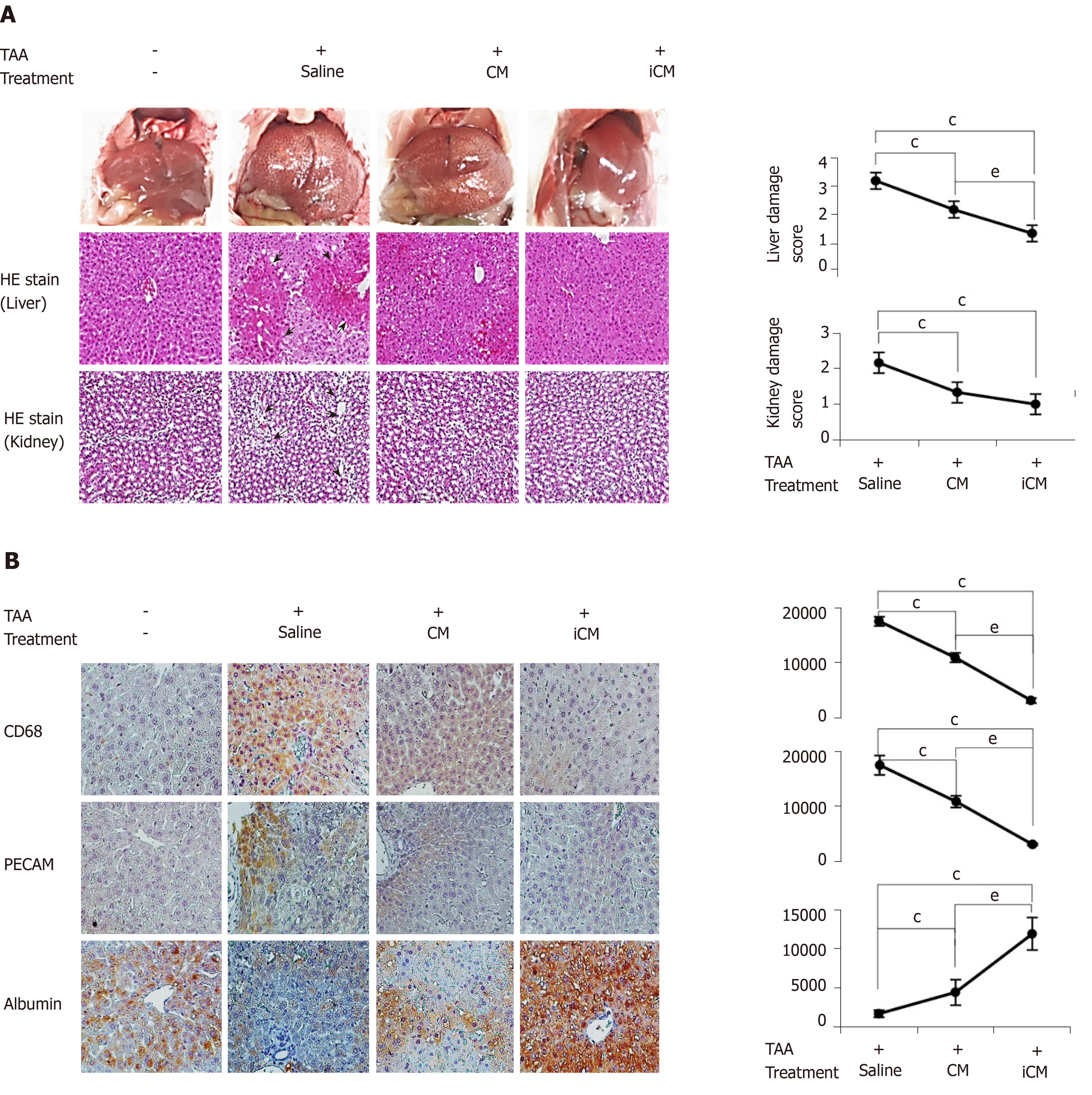
Figure 5 Various stains validating effects of thioacetamide-induced secretome in the mouse model of thioacetamide-induced hepatic failure.
A: TAA-induced conditioned media infusion significantly decreased both liver damage and kidney damage scores calculated based on hematoxylin and eosin staining; B: Immunohistochemical staining of liver specimens. Immunohistochemical stains showed that TAA-induced conditioned media infusion significantly decreased the expression of inflammatory markers (CD68 and PECAM) and significantly increased the expression of albumin. Values are presented as mean ± SD of three independent experiments. cP < 0.05 vs control (saline); eP < 0.05 between conditioned media and induced conditioned media. iCM: Induced secretome group of which components were the TAA-induced secretome; CM: Normal conditioned media group of which components were the naïve secretome; TAA: Thioacetamide.
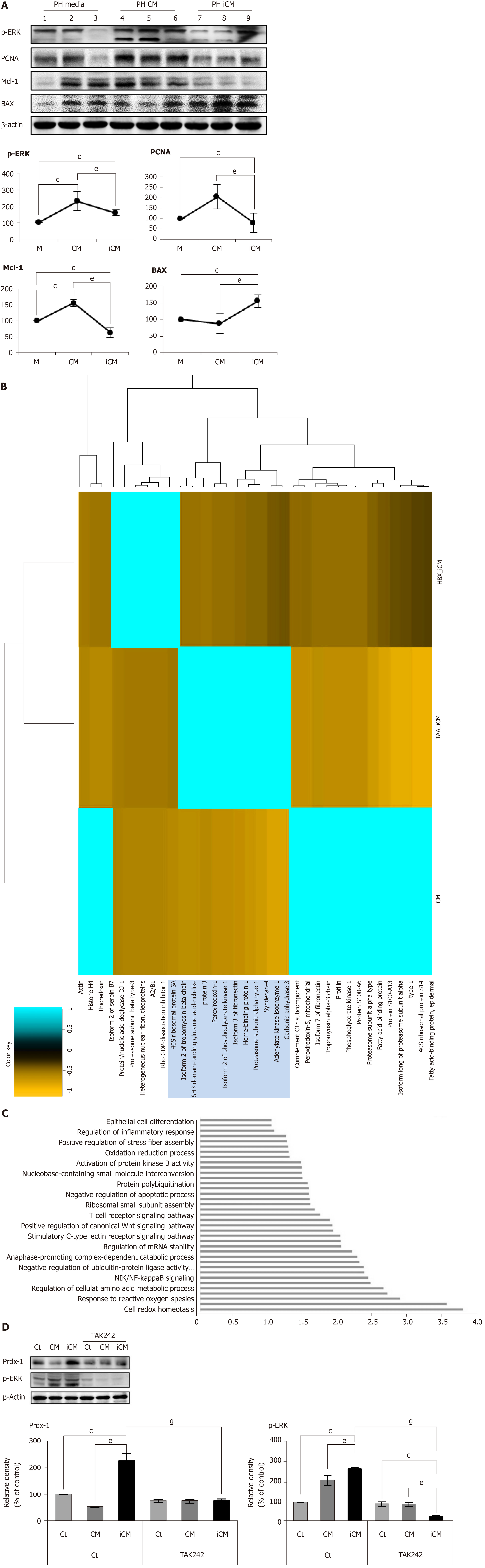
Figure 6 Validation of disease specificity and analysis of secretome components.
A: Western blot analysis showing effectiveness of the TAA-induced secretome (isecretome) in partially hepatectomized mice. TAA-induced conditioned media (iCM) infusion was inferior to CM infusion in partially hepatectomized mice, considering the lower expression of proliferation markers (p-ERK and PCNA), and an anti-apoptotic marker (Mcl-1) and higher expression of a proapoptotic marker (Bax) following TAA-iCM infusion; B: Heat map generated from label-free liquid chromatography–mass spectrometry for quantitative proteomics reflecting protein expression values of the secretome, TAA-isecretome, and HBx-isecretome. Samples are arranged in columns and proteins in rows. Red shades indicate increased expression in samples compared to in control samples; green shades indicate reduced expression; black indicates median expression. Each sample for liquid chromatography–mass spectrometry was pooled from three samples of the secretome. The components and concentration of various essential proteins varied widely between iCM and HBx-iCM, validating their specificity. Values are presented as mean ± SD of three independent experiments; C: Gene ontology enrichment analysis of the ten proteins that had been exclusively identified in the TAA-isecretome. Gene ontology enrichment analysis identified the 19 enriched biological networks of the 10 proteins. Of the 19 enriched biological networks, two of the most prominent biological processes were the response to reactive oxygen species and cell redox homeostasis, all of which were related to antioxidant activity; D: Peroxiredoxin-1 (Prdx-1) inhibition test. Prdx-1 is one of the potent antioxidant proteins and was found to be one of ten proteins that had been exclusively identified in the TAA-isecretome. We performed Prdx-1 inhibition test for the determination of the role of Prdx-1 in the hepatic reparative process. Herein, liver regenerative capacity was estimated by the expression of a hepatoproliferative marker (p-ERK). When treated with the TAA-iCM, AML2 hepatocytes showed increased expression of p-ERK as well as Prdx-1 compared with AML2 cells treated with the control CM (P < 0.05). Subsequently, we compared the efficacy of the TAA-iCM after pre-treatment of TAK242, a TLR4 inhibitor. Pre-treatment of TAK242 did not only inhibit the expression of Prdx-1 but also inhibited the expression of p-ERK, the hepatoproliferative marker, suggesting the hepatoprotective role of Prdx-1 in the liver. cP < 0.05 vs control (saline); eP < 0.05 between CM and iCM; gP < 0.05 between iCMs with or without TAK242. BIM: Bcl-2-like protein 11; iCM: Isecretome group of which components were the TAA-induced secretome; HBx-iCM: Isecretome group of which components were the HBx-induced secretome; Mcl-1: Myeloid cell leukemia 1; CM: Normal conditioned media group of which components were the naïve secretome; PCNA: Proliferating cell nuclear antigen; Prdx-1: Peroxiredoxin-1.
- Citation: Kim OH, Hong HE, Seo H, Kwak BJ, Choi HJ, Kim KH, Ahn J, Lee SC, Kim SJ. Generation of induced secretome from adipose-derived stem cells specialized for disease-specific treatment: An experimental mouse model. World J Stem Cells 2020; 12(1): 70-86
- URL: https://www.wjgnet.com/1948-0210/full/v12/i1/70.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i1.70