修回日期: 2014-04-08
接受日期: 2014-04-18
在线出版日期: 2014-05-28
目的: 观察注射Hepal-6细胞热休克后裂解蛋白致敏的骨髓来源树突状细胞(bone marrow derived dendritic cells, BMDCs)瘤苗对小鼠肝细胞癌(hepatocellular carcinoma, HCC)瘤内CD25+叉头盒转录因子P3(forkhead box p3, Foxp3)+调节T淋巴细胞(regulatory T cells, Tregs)浸润的影响.
方法: 在粒细胞-巨噬细胞集落刺激因子(granulocyte-macrophage colony stimulating factor, GM-CSF)和白介素-4(interleukin-4, IL-4)诱导下体外扩增BMDCs, 使用Hepal-6细胞热休克后裂解蛋白体外致敏BMDCs制备瘤苗, 荧光免疫化学染色和FACS检测致敏前后BMDCs CD11c、CCR7、CD80和CD86的表达变化. 使用Hepal-6细胞皮下注射的方法制备小鼠(C57BL/6J)HCC模型, 成瘤小鼠分组注射Hepal-6细胞热休克后裂解蛋白致敏的BMDCs瘤苗(足垫部和瘤内, 每7 d注射1次, 共2次), 并另设对照(空白对照组、BMDCs组和Hepal-6细胞裂解蛋白组). 在治疗结束后9 d获取组织标本, 免疫荧光组织化学染色和FACS检测瘤苗注射后肿瘤内CD8+ T细胞和CD25+ Foxp3+ Tregs细胞的浸润情况.
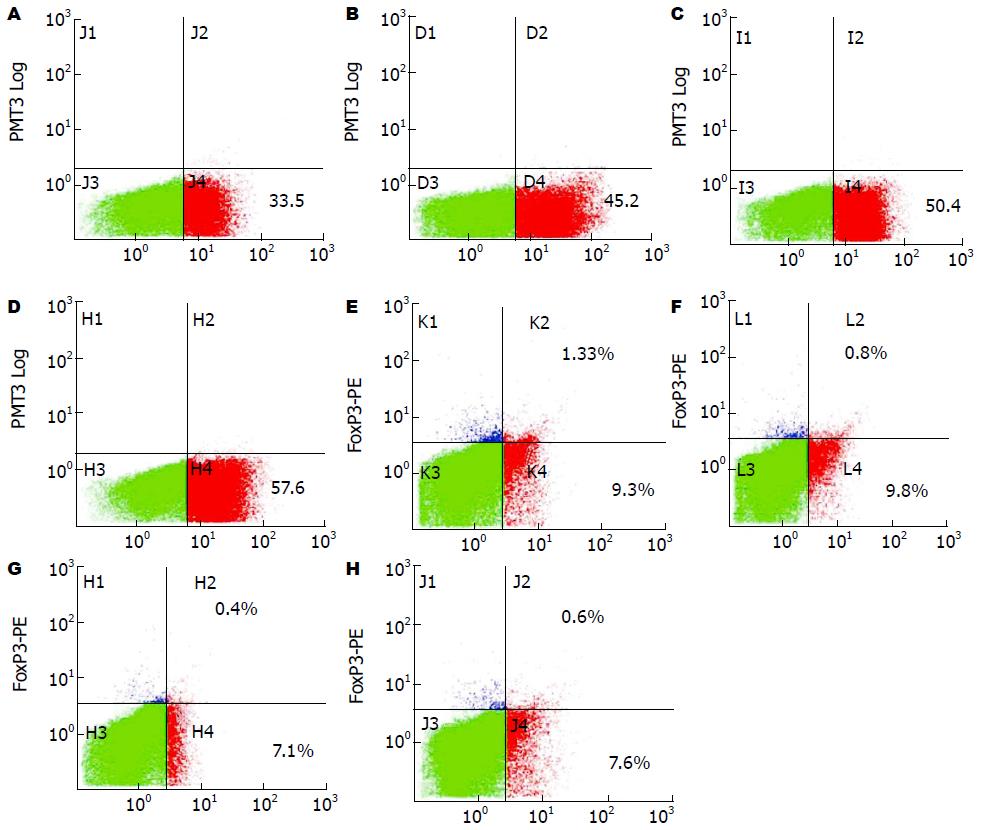
结果: 光镜和扫描电镜显示: GM-CSF和IL-4在体外诱导扩增的BMDCs具有树突状细胞特征性的形态特征, 且免疫细胞化学染色显示: 该细胞表达CD11c, CCR7, CD80和CD86. 使用Hepal-6细胞热休克后裂解蛋白致敏的BMDCs组, 与对照组(BMDCs组和Hepal-6细胞裂解蛋白组)相比该组细胞CD11c(67.2±4.49 vs 52.4±5.20, 58.4±4.43, P<0.01), CCR7(65.4±5.34 vs 45.9±5.04, 57.0±3.46, P<0.01), CD80(62.9±4.69 vs 46.9±4.75, 54.4±3.47, P<0.01)和CD86(73.3±3.58 vs 60.1±2.98, 63.7±3.10, P<0.01)的表达均明显增高. 使用Hepal-6细胞热休克后裂解蛋白致敏的BMDCs瘤苗为HCC荷瘤小鼠进行注射治疗, 治疗后的检测结果显示: 该组小鼠瘤内CD8+ T细胞的浸润明显高于对照组(空白对照组、BMDCs组和Hepal-6细胞裂解蛋白组)(55.0±4.11 vs 38.2±3.34, 44.6±4.29, 45.6±4.92, P<0.01), 而同时瘤内CD25+Foxp3+ Tregs细胞的浸润则明显低于相应对照组(0.37±0.028 vs 1.31±0.020, 0.77±0.057, 0.57±0.062, P<0.05).
结论: 使用Hepal-6细胞热休克后裂解蛋白致敏的BMDCs瘤苗进行治疗, 可增强HCC小鼠瘤内CD8+ T细胞的浸润, 并同时减少CD25+ Foxp3+ Tregs细胞的浸润, 该瘤苗具有抗肿瘤免疫效果.
核心提示: 肿瘤免疫耐受是肿瘤免疫治疗的瓶颈, 而CD4+CD25+Foxp3+ T细胞在肿瘤内的浸润是构成瓶颈的关键因素之一. 尽管不同形式的抗原刺激DCs制备的疫苗有一定的抗肿瘤效应但效果不理想, 抗原的免疫原性是影响疫苗效果的关键因素之一. 本研究利用Hepal-6细胞热休克后的裂解蛋白结合了肿瘤细胞裂解蛋白全抗原性和热休克后免疫原性增强的优势, 在体外有较强致敏DCs成熟的作用, 在体内能活化和促进CD8+ T细胞迁移入肿瘤灶, 减少CD4+CD25+Foxp3+ T细胞在瘤内的浸润, 有较强的抗肿瘤效应.
引文著录: 李日伦, 周爽, 秦杰, 梁春敏, 罗国容. Hepal-6细胞热休克后裂解蛋白致敏的树突状细胞瘤苗对肝细胞癌瘤内CD25+Foxp3+ Treg细胞的影响. 世界华人消化杂志 2014; 22(15): 2081-2090
Revised: April 8, 2014
Accepted: April 18, 2014
Published online: May 28, 2014
AIM: To determine whether the bone marrow derived dendritic cell (BMDC) vaccine sensitized by heat shocked hepal-6 cell proteins affects the infiltration of intratumoral CD25+Foxp3+ Tregs in a mouse hepatocellular carcinoma (HCC) model.
METHODS: In the presence of GM-CSF and IL-4, BMDCs were induced in vitro. BMDCs were sensitized by heat shocked hepal-6 cell proteins to generate a vaccine for HCC. The expression of CD11c, CCR7, CD80 and CD86 on these sensitized BMDCs were analyzed by FACS. The anti-tumor effect of this vaccine was evaluated using a mouse HCC model established by subcutaneous injection of Hepal-6 cells. Eight days later, the tumor-bearing mice were divided into four groups, which underwent intratumoral injection of BMDCs sensitized by heat shocked hepal-6 cell proteins, serum-free culture medium, BMDCs without sensitization and BMDCs sensitized by unheated hepal-6 cell proteins (once every 7 d, 2 times altogether), respectively. Nine days after final administration, the mice were sacrificed and the tumor samples were taken for immunofluorescence staining for CD8+ cells and intratumoral CD25+Foxp3+ Tregs.
RESULTS: Light microscopy and scanning electron microscopy showed that BMDCs propagated in the presence of GM-CSF and IL-4 displayed the typical morphological characteristics of dendritic cells. Immunocytochemical staining showed that they expressed the dendritic cell marks including CD11c, CCR7, CD80 and CD86. Compared with the controls (BMDCs without sensitization or sensitized by unheated hepal-6 cells proteins), the BMDCs sensitized by heat shocked hepal-6 cells proteins showed increased expression of CD11c (67.2 ± 4.49 vs 52.4 ± 5.20, 58.4 ± 4.43), CCR7 (65.4 ± 5.34 vs 45.9 ± 5.04, 57.0 ± 3.46), CD80 (62.9 ± 4.69 vs 46.9 ± 4.75, 54.4 ± 3.47) and CD86 (73.3 ± 3.58 vs 60.1 ± 2.98, 63.7 ± 3.10) (P < 0.01 for all). Compared with the controls, the mice administrated with the BMDC vaccine sensitized by heat shocked Hepal-6 cell proteins showed increased CD8+ T cells (55.0 ± 4.11 vs 38.2 ± 3.34, 44.6 ± 4.29, 45.6 ± 4.92, P < 0.01 for all) and decreased intratumoral CD25+Foxp3+ Tregs (0.37 ± 0.028 vs 1.31 ± 0.020, 0.77 ± 0.057, 0.57 ± 0.062, P < 0.05 for all).
CONCLUSION: Heat shocked hepal-6 cell protein sensitization can upregulate the expression of CD11c, CCR7, CD80 and CD86 on BMDCs in vitro. Administration with this BMDC vaccine can increase CD8+ T cells and decrease intratumoral CD25+Foxp3+ Tregs in HCC mice.
- Citation: Li RL, Zhou S, Qin J, Liang CM, Luo GR. Effect of administration of BMDC vaccine sensitized by heat shocked hepal-6 cell proteins on intratumoral CD25+Foxp3+ Tregs in mouse hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi 2014; 22(15): 2081-2090
- URL: https://www.wjgnet.com/1009-3079/full/v22/i15/2081.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i15.2081
肿瘤是严重威胁人类健康和生命的常见疾病. 肿瘤发生、发展的原因复杂, 研究表明, 肿瘤宿主体内普遍存在由肿瘤细胞相关抗原、肿瘤浸润淋巴细胞(tumor-infiltrating lymphocytes, TILs)和细胞因子等组成的免疫耐受微环境[1]. 调节T淋巴细胞(regulatory T cells, Tregs)是TILs的成员之一[2], 肿瘤患者外周血和肿瘤灶CD4+CD25+叉头盒转录因子P3(forkhead box p3, Foxp3)+ Tregs浸润普遍增多[3-5]. Tregs可抑制辅助T淋巴细胞-1(T helper type 1, Th1)增殖, 下调Th1细胞介导的抗肿瘤免疫[6,7]. 因此, 提高肿瘤的免疫原性、促进免疫系统抗肿瘤排斥反应是肿瘤免疫治疗的重要手段. 肿瘤免疫治疗的关键一环是促进树突状细胞(dendritic cells, DCs)的成熟与提高其抗原呈递能力[8]. DCs是功能强大的专职抗原呈递细胞[8], 利用肿瘤或肿瘤细胞裂解物[9,10]、肿瘤RNA[11]和细胞因子[12]等致敏的DCs瘤苗已对一些肿瘤取得免疫治疗效果. 研究已证实, 肿瘤细胞裂解蛋白具有全抗原性, 致敏的DCs能活化宿主体内多克隆T细胞产生抗肿瘤免疫效应[9,10]. 肿瘤细胞热休克后产生大量具有强抗原性的热休克蛋白(heat shocked protein, HSP), 如HSP70和HSP90等, 具有提高细胞抗原性的作用[13]. 本研究结合肿瘤细胞裂解蛋白的全抗原性和热休克后抗原性提高的双重优点, 利用Hepal-6细胞热休克后裂解蛋白体外致敏BMDCs制成瘤苗, 每隔7 d足垫部和瘤内注射HCC小鼠共2次, 另设空白组、BMDCs组、Hepal-6细胞裂解蛋白致敏BMDCs组为对照. 治疗结束后第9 d处死各组小鼠, FACS检测肿瘤组织内CD8+T细胞及CD25+ Foxp3+细胞浸润的改变.
6-8周龄C57BL/6J近交系♀小鼠从中国科学院上海实验动物中心购买; Hepal-6为小鼠来源的肝癌细胞株(ATCC CRL-1830), 由复旦大学附属中山医院肝癌研究所保存并提供; 高糖DMEM、RPMI 1640、胰蛋白酶(trypsin)-EDTA、胎牛血清(heat-inactivated fetal calf serum)购自GIBCOL公司; 培养板和离心管、细胞培养瓶从Corning Corstar公司购买; PE-conjugated rat anti mouse CD11c(N418)、CCR7(4B12), FITC-conjugated rat anti mouse CD80(16-10A1)、CD86(GL1)、FITC-conjugated rat anti mouse CD25(PC61.5)、PE-conjugated rat anti mouse Foxp3(FJK16s)、FITC-conjugated rat anti mouse CD8、affinity purified rat anti mouse CD25 (PC61.5)、affinity purified rabbit anti mouse Foxp3 (NRRF-30)购自eBioScience公司; FITC-conjugated goat anti rat IgG、Cy3-conjugated goat anti rabbit IgG购自KPL公司; Mouse CD4+ CD25+ Regulatory T cell Isolation Kit购自Miltenyi Biotec公司.
1.2.1 Hepal-6细胞培养和传代: (1)从液氮罐取出Hepal-6 细胞冻存管快速放入37 ℃无菌双蒸水液化后, 吸出冻存液放入DMEM:RPMI 1640 = 1:1混合培养液(10%胎牛血清)600 r/min离心6 min; (2)将细胞放入盛有DMEM:RPMI 1640 = 1:1混合培养液(10%胎牛血清)培养瓶, 5%CO2、37 ℃饱和湿度培养箱培养; (3)显微镜下观察贴壁生长的细胞基本铺满瓶底时倒弃培养液, 加入0.25%的胰蛋白酶室温消化后, 600 r/min离心6 min, 重悬细胞. 按1.0×105/mL细胞浓度分瓶加入DMEM:RPMI 1640 = 1:1混合培养液(10%胎牛血清)继续培养, 根据细胞生长情况和培养液颜色变化更换培养液.
1.2.2 Hepal-6肿瘤模型的制作: (1)Hepal-6细胞传代3次后收集对数生长期细胞, 0.25%的胰蛋白酶-EDTA消化, 600 r/min离心6 min, 重悬并调整细胞浓度为2×107/mL; (2)按实验分组要求取C57BL/6J小鼠乙醚轻微麻醉后, 每只右肩胛区皮下1次性接种0.2 mL浓度为2×107/mL的Hepal-6细胞, 按正常条件饲养, 在接种第7-8 d小鼠右肩胛区可触及直径约5-7 mm的肿瘤.
1.2.3 两步法免疫磁珠体外分离小鼠肿瘤CD4+CD25+ T细胞: 参考试剂盒操作: (1)成瘤12 d摘荷瘤小鼠肿瘤, 1×PBS碾磨肿瘤组织后, 4 ℃、1000 r/min离心10 min; (2)细胞混匀, 加入9 mL双蒸水5-7 s后, 迅速加入1 mL 10×PBS终止破膜, 1×PBS重悬细胞; (3)加台盼蓝染液显微镜下观察和计数细胞, 细胞活力>90%时继续后续步骤; (4)去除非CD4+ T细胞: 将细胞1000 r/min 4 ℃离心10 min, 每107细胞加40 µL PBA和10 µL cook-tail-Biotin antibody混匀细胞, 4 ℃孵育15 min; 加30 µL PBA、20 µL Anti-Biotin MicroBeads和10 µL CD25-PE antibody再次混匀细胞, 4 ℃避光孵育20 min; 加入1 mL PBA, 1000 r/min 4 ℃离心10 min后加500 µLPBA混匀; 加2 mL PBA润洗LD柱中后1次性加入上述500 µL细胞悬液, 收集流出物即为CD4+ T细胞; (5)标记分离的CD4+CD25+ T细胞: 将收集的CD4+ T细胞1000 r/min 4 ℃离心10 min, 90 µL PBA和10 µL Anti-PE MicroBeads混匀细胞; 4 ℃避光孵育20 min后加2 mL PBA 1000 r/min 4 ℃离心10 min后加500 µL PBA重悬细胞; 用500 µL PBA润洗MS柱后, 将上述500 µL细胞悬液1次性加入MS柱中, 流出的为CD4+CD25- T细胞, 留在MS柱中的为CD4+CD25+ T细胞.
1.2.4 CD25+和Foxp3+抗体免疫荧光法双标分离的CD4+CD25+ T细胞: (1)将分离的CD4+CD25+ T细胞制作细胞涂片自然干燥后冷丙酮固定20 min, 1×PBS漂洗3次; (2)0.1%Triton-X-100室温下处理10 min, 1×PBS漂洗3次, 山羊血清室温封闭30 min; (3)滴加兔抗小鼠Foxp3单克隆抗体, 大鼠抗小鼠CD25抗体4 ℃孵育过夜后1×PBS漂洗3次; (4)滴加Cy3-羊抗兔IgG, FITC-羊抗大鼠IgG室温避光孵育2 h, 加入1:1000稀释的DAPI液, 室温避光孵育, 1×PBS漂洗3-4次后磷酸甘油封片送至中科院上海细胞研究所行激光共聚焦显微镜拍照.
1.2.5 Hepal-6细胞热休克与蛋白的提取: (1)将传3代的Hepal-6细胞换培养液后继续培养12 h; (2)先将培养箱在5%CO2环境下缓慢升温至42 ℃, 放入细胞热休克1 h后立即取出在5%CO2、37 ℃培养箱中恢复6 h取出细胞, 细胞刷小心刮取瓶底细胞, 1×PBS洗涤3次; (3)双蒸水重悬细胞至-80 ℃冰箱冻融3次后研磨细胞, 将细胞研磨液14000 r/min、4 ℃离心30 min, 小心吸取蛋白上清, 即为总蛋白, 分装在-80 ℃冰箱保存. BCA法测定蛋白质的浓度.
1.2.6 BMDCs的诱导培养: (1)处死6-8周龄C57BL/6J小鼠, 取股骨剔除所附肌肉, 用1次性注射器在1×PBS中冲出骨髓至股骨发白; (2)收集细胞悬液, 1000 r/min离心10 min, 加9 mL双蒸水约5-10 s破除红细胞后随即加1 mL 10×PBS中止; (3)细胞过滤后1000 r/min 离心10 min, 细胞计数后调整细胞浓度至2×106/mL. 以2×106细胞/孔接种到含重组小鼠GM-CSF(20 ng/mL)和IL-4(10 ng/mL)的24孔培养板, 5%CO2、37 ℃培养, 隔天半量换培养液并补足细胞因子继续培养.
1.2.7 BMDCs的鉴定: (1)相差显微镜观察并拍照培养BMDCs集落的大小、数量和突起等; (2)收集培养9 d的BMDCs, 1×PBS 1000 r/min离心10 min洗涤3次. 部分送复旦大学上海医学院电镜室扫描电镜拍照, 部分制成细胞涂片; (3)冷丙酮固定细胞涂片20 min后一部分HE染色并拍照, 一部分用于荧光免疫组织化学检测CD11c、CCR7、CD80、CD86的表达.
1.2.8 Hepal-6细胞热休克后裂解蛋白致敏BMDCs: (1)将培养5 d的BMDCs半量换培养液并调整细胞浓度至2×106/mL, 5%CO2、37 ℃培养箱培养24 h后分3组: BMDCs对照组、Hepal-6细胞裂解蛋白BMDCs对照组、脂多糖(lipopolysaccharides, LPS)(200 ng/mL)刺激对照组和Hepal-6细胞热休克后裂解蛋白BMDCs实验组. 每组设6复孔; (2)BMDCs对照组每孔加入等体积的1×PBS; Hepal-6细胞裂解蛋白BMDCs对照组每孔加入1.5 μg Hepal-6细胞裂解蛋白; Hepal-6细胞热休克后裂解蛋白BMDCs组每孔加入1.5 μg Hepal-6细胞热休克后裂解蛋白; (3)各孔培养液均调至1 mL, 5%CO2、37 ℃培养12 h后, 收集各组细胞并调整细胞浓度至2×107/mL.
1.2.9 FACS分析Hepal-6细胞热休克后裂解蛋白致敏前后BMDCs的CD11c、CCR7、CD80、CD86表达: (1)将收集的各组BMDCs各分装5个EP管, 2000 r/min离心3 min; (2)各EP单独加500 μL含PE-大鼠抗小鼠CCR7、CD11c单克隆抗体, FITC-大鼠抗小鼠CD80、CD86单克隆抗体, 4 ℃避光孵育40 min; (3)1×PBS, 2000 r/min离心3 min, 洗2次加500 μL Flow Cytometry Staining Buffer重悬混匀, 上机检测, 用随机所带流式分析软件分析实验数据.
1.2.10 Hepal-6细胞热休克后裂解蛋白致敏BMDCs注射荷瘤小鼠: 接种8 d后成瘤小鼠随机分4组. (1)荷瘤对照组: 每只小鼠瘤内和足垫部各注射0.1 mL 1×PBS; (2)对照BMDCs组每只小鼠瘤内和足垫部各注射0.1 mL浓度为2×107/mL的BMDCs; (3)Hepal-6细胞裂解蛋白致敏BMDCs组: 每只小鼠瘤内和足垫部各注射0.1 mL浓度为2×107/mL的Hepal-6细胞裂解蛋白致敏BMDCs; (4)Hepal-6细胞热休克后裂解蛋白致敏BMDCs组: 每只小鼠瘤内和足垫部各注射0.1 mL浓度为2×107/mL的Hepal-6细胞热休克后裂解蛋白致敏BMDCs, 各组每7 d注射1次, 共2次.
1.2.11 FACS分析小鼠肿瘤CD8+ T细胞和CD25+Foxp3+ T细胞的浸润: (1)治疗9 d后, 处死各组小鼠, 取肿瘤组织并辗碎, PBS冲洗成细胞悬液1000 r/min离心10 min; (2)加9 mL双蒸水轻摇5-8 min破除红细胞膜迅速加1 mL 10×PBS终止, 1000 r/min离心10 min; (3)细胞计数, 调细胞浓度至1×106/mL; (4)将各组细胞2000 r/min离心3 min, 分别加100 μL Flow Cytometry Staining Buffer后各加入: FITC-大鼠抗小鼠CD8抗体、PE-大鼠抗小鼠Foxp3+抗体和FITC-大鼠抗小鼠CD25抗体混匀, 4 ℃避光孵育40 min; (5)1 mL 1×PBS, 2000 r/min离心3 min洗涤2次后, 各加500 μL Flow Cytometry Staining Buffer重悬混匀, 上机检测, 用随机所带流式分析软件分析实验数据.
统计学处理 实验数据以mean±SD表示, 组间差异采用SPSS16.0统计软件进单因素方差分析, 若方差齐性, 两两比较采用LSD法, 若方差不齐, 采用Games-Howell检验分析. P<0.05为差异有统计学意义.
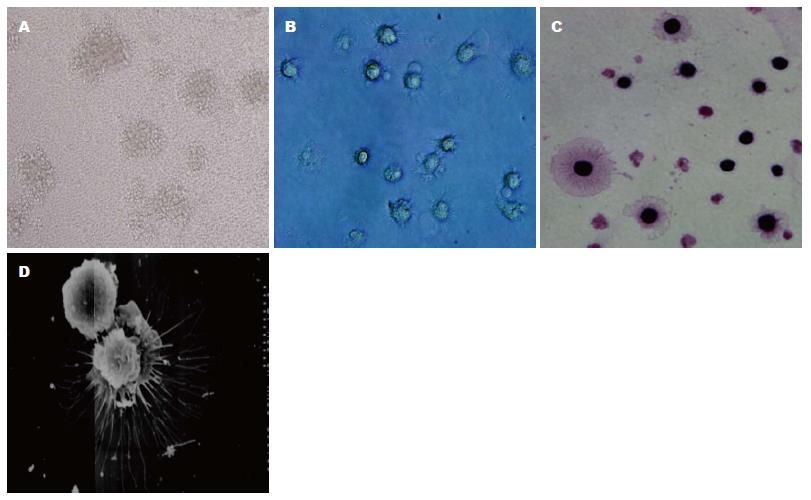
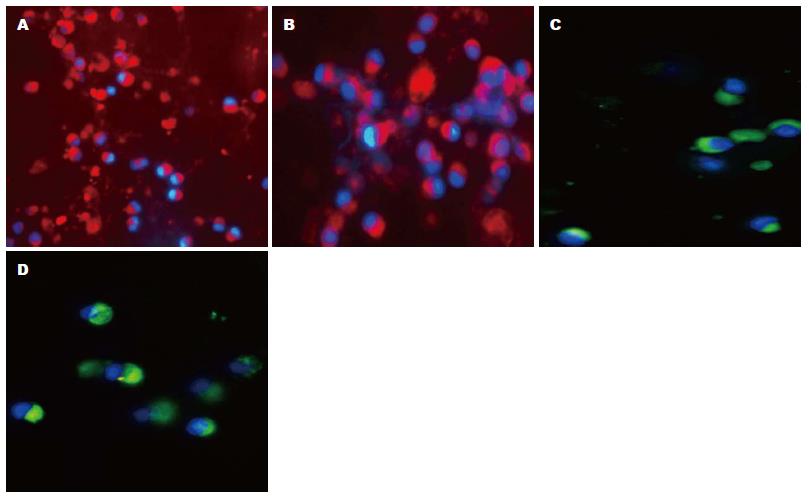
显微镜下观察可见: GM-CSF和IL-4体外诱导的C57BL/6J小鼠BMDCs在第3天形成明显的集落, 第6天集落增大, 并释放出较多的BMDCs, 第9、12天集落释放的BMDCs数量更多, 集落松散变小. HE染色显示第9天的BMDCs有明显树突状突起, 扫描电镜可见多数次级分支(图1), 荧光免疫细胞化学染色显示: BMDCs表达CD11c、CCR7、CD80和CD86(图2).
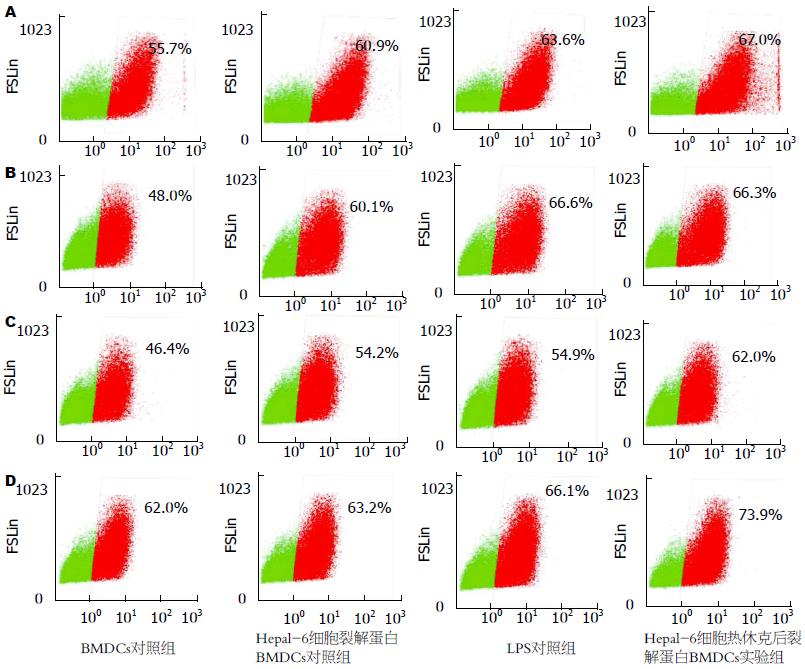
FACS分析显示(表1, 图3): Hepal-6细胞热休克后裂解蛋白致敏的BMDCs上调了CD11c、CCR7、CD80和CD86的表达, 与BMDCs对照组和Hepal-6细胞裂解蛋白对照组相比差异具有统计学意义(P<0.01). 尽管LPS刺激同样可以上调BMDCs CD11c、CCR7、CD80和CD86的表达(与Hepal-6细胞裂解蛋白组相比, P<0.05), 但Hepal-6细胞热休克后裂解蛋白致敏的BMDCs比LPS组具有更高的CD80和CD86表达, 且两组之间的差异具有统计学意义(CD80: P<0.05, CD86: P<0.01).
在对HCC模型小鼠运用瘤苗进行治疗后的第9天(图4), 针对瘤内CD8+ T细胞和CD25+Foxp3+ Tregs细胞浸润情况的FACS检测显示(表2, 图5): 与空白对照组、BMDCs对照组和Hepal-6细胞裂解蛋白致敏BMDCs对照组比, Hepal-6细胞热休克后裂解蛋白致敏的BMDCs组呈现增强的CD8+ T细胞瘤内浸润(P<0.01), 但该组瘤内CD25+Foxp3+ Tregs细胞的浸润则明显减少(P<0.01或P<0.05).
肿瘤微环境的免疫耐受表现包括抗原缺失、免疫抑制细胞增多[3]、DCs抗原提呈能力下降等[14-17]. 因此, 有效的抗瘤免疫治疗首先要有强免疫原刺激DCs成熟, 促进其抗原呈递能力. 其次是成熟的DCs在荷瘤宿主体内能促进免疫效应CD4+ T细胞、CD8+ T细胞的增殖和活化, 抑制和减少Tregs等免疫抑制细胞在瘤内的浸润. Tregs是以免疫抑制为主要功能的T细胞, 表达CD25[18]和Foxp3分子[19], Foxp3的是Tregs活化与功能成熟的标志[20], Foxp3表达能复苏休眠状态的Tregs, 提高其增殖潜能[21]. CD25+Foxp3+ Tregs在多种肿瘤浸润[22], 且与结肠癌[23,24]、结直肠癌[25]、胃癌[26]、头颈癌[27,28]、淋巴瘤[29]、宫颈癌[4]、卵巢癌[30]和肺癌[31]等预后相关. 肿瘤浸润的CD25+Foxp3+ Tregs抑制CD4+ T细胞、CD8+ T细胞的活化、增殖和细胞因子的分泌[32], 抑制自然杀伤细胞(natural killer, NK)细胞和DCs的成熟[33], 抑制B细胞的增殖和抗体产生[34]. CD25+ Foxp3+ Tregs的免疫抑制机制主要是细胞直接接触的细胞毒T细胞相关抗原4(cytotoxic T lymphocyte antigen-4, CTLA-4)途径[35,36]和细胞因子白介素-10(interleukin-10, IL-10)[37]、转化生长因子-β1(transforming growth factor-β1, TGF-β1)途径[38].
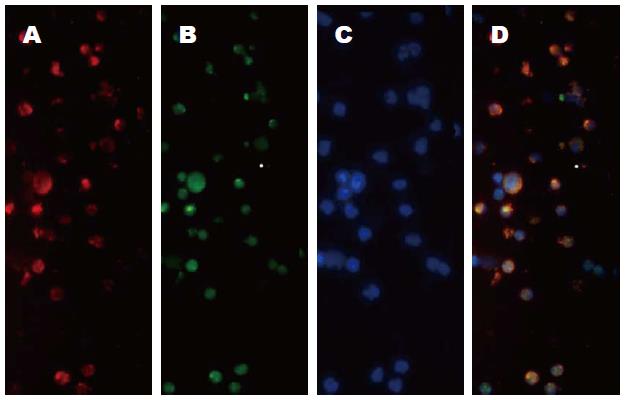
本实验免疫荧光组织化学双标法显示Hepal-6细胞建立的HCC小鼠瘤内有CD25+Foxp3+ Tregs浸润(图4), 提示CD25+Foxp3+ Tregs与小鼠HCC关系密切. 实验诱导的BMDCs在显微镜下具有典型的DCs集落和树突, 表达CCR7、CD11c、CD80和CD86分子. Hepal-6细胞热休克后裂解蛋白致敏BMDCs实验组上调CD11c、CCR7、CD80 、CD86的表达(P<0.01); DCs成熟刺激物质LPS对照组BMDCs的CD11c、CCR7、CD86的表达也上调(P<0.05), 实验组CD80(P<0.05)、CD86(P<0.01)表达高于LPS组, 提示Hepal-6细胞热休克后裂解蛋白具有较强的刺激BMDCs成熟能力. 致敏后的BMDCs瘤苗足垫部和瘤体内注射治疗HCC小鼠后, 明显下调CD25+Foxp3+ Tregs(P<0.05), 但上调CD8+ T细胞在瘤体内的浸润(P<0.01). 提示瘤苗对瘤体CD8+ T细胞的浸润有增强作用, 但对CD25+Foxp3+ Tregs浸润有抑制作用. Hepal-6细胞热休克后裂解蛋白对BMDCs的成熟刺激以及疫苗对瘤体CD25+Foxp3+ Tregs和CD8+ T细胞浸润的影响可能与以下机制有关: Hepal-6热休克后裂解蛋白的多种抗原成分被BMDCs捕获并被处理, 以MHC-Ⅱ类分子或抗原肽表达于细胞膜[39-41], HSPs、HSPs-抗原肽复合物与BMDCs表面的CD40、CD91结合被内化促进BMDCs成熟为DCs(mature dendritic cells, mDCs)[42]. mDCs上调表达CCR7、CD80和CD86分子[39], CCR7与次级淋巴组织趋化因子(secondary lymphoid chemokin, SLC)结合后一方面提高DCs的趋化迁移能力有利于抗原递呈, 另外一方面在宿主体内趋化周围淋巴器官的CD4+ T细胞、CD8+ T细胞迁移入肿瘤发挥抗瘤作用[43]. mDCs在体内能促进CD8+ T细胞的归巢和活化[44-46], 下调TGF-β、IL-10表达抑制CD25+ Foxp3+ Tregs的增殖和功能[9,10,12,18,19], 减弱Foxp3+ Tregs的免疫抑制作用[38].
总之, Hepal-6细胞热休克后裂解蛋白体外能促进BMDCs的成熟, 疫苗注射治疗后能抑制CD25+Foxp3+ Tregs但增强CD8+ T细胞在HCC小鼠肿瘤的浸润, 改善免疫环境, 促进肿瘤免疫排斥, 有明显的抗肿瘤效应, 但疫苗的作用机制还要有待实验进一步阐明.
肿瘤微环境是肿瘤发生和发展的重要环境, 也是肿瘤免疫耐受的主要因素. 肿瘤免疫耐受主要表现为抗原缺失、CD25+Foxp3+ T细胞增多和树突状细胞(dendritic cells, DCs)功能下降等. 因此, 寻求免疫原强的抗原有效促进DCs成熟与抗原呈递能力, 减弱CD25+ Foxp3+细胞功能, 促进CD8+ T细胞的增殖和活化是肿瘤免疫治疗的研究热点.
代智, 副研究员, 复旦大学附属中山医院肝癌研究所
肿瘤裂解物、肿瘤细胞裂解蛋白、肿瘤RNA、细胞因子等致敏或基因修饰DCs等制作抗肿瘤疫苗是肿瘤免疫治疗的重要手段. 但迄今还未找到理想的刺激DCs成熟的抗原物质, 因此DCs疫苗的开发仍是探索的主要方向.
研究表明, 肿瘤细胞裂解蛋白具有全抗原性, 能刺激多个T细胞表位而活化多克隆T细胞, 热休克蛋白(heat shocked protein, HSP)在多数肿瘤不同程度表达异常, HSP能将与其结合的多肽通过内源性途径经MHC-Ⅰ类分子而引起特异的CD8+ T细胞反应. 因此, 利用肿瘤细胞裂解蛋白的全抗原性和HSP或HSP-肽结合物免疫原性强的联合作用有可能刺激更强的抗仲瘤免疫反应.
DCs疫苗的制备多用单一抗原或单个基因修饰, 抗瘤效果不理想. 本研究利用肿留裂解蛋白的全抗原性结合肿瘤细胞热休克后HSP表达上调, 免疫原性增强的优点以制备免疫效果更强的DCs疫苗.
Hepal-6细胞热休克后裂解蛋白体外对骨髓来源树突状细胞(bone marrow derived dendritic cells, BMDCs)有较强的致敏作用, 致敏的BMDCs在体内能促进小鼠肝细胞癌肿瘤灶内CD8+ T细胞的增殖和活化, 减少CD4+ CD25+Foxp3+ T细胞的浸润, 对肝癌的免疫治疗有参考作用.
文章新颖性突出, 对读者有一定的参考价值.
编辑: 郭鹏 电编:鲁亚静
| 1. | Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263-274. [PubMed] [DOI] |
| 2. | Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233-240. [PubMed] [DOI] |
| 3. | Esendagli G, Bruderek K, Goldmann T, Busche A, Branscheid D, Vollmer E, Brandau S. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer. 2008;59:32-40. [PubMed] [DOI] |
| 4. | Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, van der Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028-2035. [PubMed] [DOI] |
| 5. | Anz D, Mueller W, Golic M, Kunz WG, Rapp M, Koelzer VH, Ellermeier J, Ellwart JW, Schnurr M, Bourquin C. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer. 2011;129:2417-2426. [PubMed] [DOI] |
| 6. | Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520-529. [PubMed] [DOI] |
| 7. | Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287-302. [PubMed] [DOI] |
| 8. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [PubMed] [DOI] |
| 9. | Fields RC, Shimizu K, Mulé JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482-9487. [PubMed] [DOI] |
| 10. | Geiger J, Hutchinson R, Hohenkirk L, McKenna E, Chang A, Mulé J. Treatment of solid tumours in children with tumour-lysate-pulsed dendritic cells. Lancet. 2000;356:1163-1165. [PubMed] [DOI] |
| 12. | Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, Oh S, Fay J, Pascual V, Banchereau J. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199-212. [PubMed] [DOI] |
| 13. | Okamoto M, Tazawa K, Kawagoshi T, Maeda M, Honda T, Sakamoto T, Tsukada K. The combined effect against colon-26 cells of heat treatment and immunization with heat treated colon-26 tumour cell extract. Int J Hyperthermia. 2000;16:263-273. [PubMed] [DOI] |
| 14. | Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989-2003. [PubMed] [DOI] |
| 15. | Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005-2016. [PubMed] [DOI] |
| 16. | Chang LL, Wang SW, Wu IC, Yu FJ, Su YC, Chen YP, Wu DC, Kuo CH, Hung CH. Impaired dendritic cell maturation and IL-10 production following H. pylori stimulation in gastric cancer patients. Appl Microbiol Biotechnol. 2012;96:211-220. [PubMed] [DOI] |
| 17. | Ananiev J, Gulubova MV, Manolova IM. Prognostic significance of CD83 positive tumor-infiltrating dendritic cells and expression of TGF-beta 1 in human gastric cancer. Hepatogastroenterology. 2011;58:1834-1840. [PubMed] [DOI] |
| 18. | Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245-1253. [PubMed] |
| 19. | Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727-3735. [PubMed] [DOI] |
| 20. | Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11:594-598. [PubMed] [DOI] |
| 21. | Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359-368. [PubMed] [DOI] |
| 22. | Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57-117. [PubMed] [DOI] |
| 23. | Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol. 2010;38:76-87. [PubMed] [DOI] |
| 24. | Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270-1279. [PubMed] [DOI] |
| 25. | Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635-2643. [PubMed] [DOI] |
| 26. | Kashimura S, Saze Z, Terashima M, Soeta N, Ohtani S, Osuka F, Kogure M, Gotoh M. CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer. 2012;15:144-153. [PubMed] [DOI] |
| 27. | Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465-472. [PubMed] [DOI] |
| 28. | Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957-2964. [PubMed] [DOI] |
| 29. | Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, Ryder LP, Ralfkiaer E. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512-2518. [PubMed] [DOI] |
| 30. | Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, Gilks CB, Clarke B, Köbel M, Nelson BH. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. [PubMed] [DOI] |
| 31. | Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH, Patz EF. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866-2872. [PubMed] [DOI] |
| 32. | Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008-1011. [PubMed] [DOI] |
| 33. | Ralainirina N, Poli A, Michel T, Poos L, Andrès E, Hentges F, Zimmer J. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144-153. [PubMed] [DOI] |
| 34. | Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446-19451. [PubMed] [DOI] |
| 35. | Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752-6761. [PubMed] |
| 36. | Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676-4680. [PubMed] |
| 37. | Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40-44. [PubMed] |
| 38. | Wan YY, Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev. 2006;212:114-130. [PubMed] [DOI] |
| 39. | Jin P, Han TH, Ren J, Saunders S, Wang E, Marincola FM, Stroncek DF. Molecular signatures of maturing dendritic cells: implications for testing the quality of dendritic cell therapies. J Transl Med. 2010;8:4. [PubMed] [DOI] |
| 40. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [PubMed] [DOI] |
| 41. | Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723-2730. [PubMed] |
| 42. | Singh-Jasuja H, Hilf N, Arnold-Schild D, Schild H. The role of heat shock proteins and their receptors in the activation of the immune system. Biol Chem. 2001;382:629-636. [PubMed] [DOI] |
| 43. | Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134-140. [PubMed] [DOI] |
| 44. | Seki I, Suzuki M, Miyasaka N, Kohsaka H. Expression of CD45 isoforms correlates with differential proliferative responses of peripheral CD4+ and CD8+ T cells. Immunol Lett. 2010;129:39-46. [PubMed] [DOI] |
| 45. | Kalinski P, Urban J, Narang R, Berk E, Wieckowski E, Muthuswamy R. Dendritic cell-based therapeutic cancer vaccines: what we have and what we need. Future Oncol. 2009;5:379-390. [PubMed] [DOI] |
| 46. | Mempel TR, Bauer CA. Intravital imaging of CD8+ T cell function in cancer. Clin Exp Metastasis. 2009;26:311-327. [PubMed] [DOI] |