修回日期: 2012-11-20
接受日期: 2012-12-17
在线出版日期: 2012-12-28
目的: 探讨Ⅱa期(pT3N0M0)结肠癌的预后因素.
方法: 收集2004-01/2008-06北京大学肿瘤医院外科手术治疗且未接受辅助化疗的Ⅱ期结肠癌患者共161例, 选取符合标准的84例Ⅱa期患者纳入研究. 回顾性分析这些患者的临床病理资料、血清学肿瘤标志物指标、免疫组织化学结果以及3年无病生存率(3 years disease free survival, 3yrDFS), 通过单因素及多因素生存分析, 探讨Ⅱa期结肠癌的预后因素.
结果: 84例入组患者的3yrDFS为88.1%. 术前血清癌胚抗原(carcinoembryonic antigen, CEA)水平升高患者的3yrDFS为77.1%, 而CEA水平正常患者的3yrDFS为95.9%, 二组间差异显著(P = 0.007). 多因素生存分析显示: 男(P = 0.024, OR = 7.212, 1.293-40.237)、术前CEA水平升高(P = 0.012, OR = 8.013, 1.573-40.817)、术前贫血(P = 0.011, OR = 6.461, 1.537-27.151)以及Ki67高表达(P = 0.099, OR = 3.298, 0.799-13.610)是提示Ⅱa期结肠癌患者预后不良的独立风险因素, 而术前血清CEA水平升高合并Ki67高表达者预后较差(3yrDFS 70%).
结论: 术前血清CEA水平联合肿瘤组织Ki67表达可以作为预测Ⅱa期结肠癌患者预后的重要指标. 对于术前血清CEA水平升高并且Ki67高表达的Ⅱa期结肠癌患者可能应采取更积极的辅助治疗.
引文著录: 王林, 顾晋, 彭亦凡. Ⅱa期结肠癌的预后因素. 世界华人消化杂志 2012; 20(36): 3816-3821
Revised: November 20, 2012
Accepted: December 17, 2012
Published online: December 28, 2012
AIM: To investigate the prognostic factors of stage Ⅱa (pT3N0M0) colon cancer.
METHODS: The demographic, clinical and laboratory data for 161 patients with stage Ⅱa colon cancer treated with curative surgery alone at Peking University Cancer Hospital from January 2004 to June 2008 were reviewed retrospectively. Eighty-four valid cases were selected in this study based on inclusive and exclusive criteria. The 3-year disease-free survival (DFS) was tested by univariate and multivariate analyses to identify prognostic factors.
RESULTS: The overall 3-year DFS for the selected 84 cases was 88.1%. The 3-year DFS for patients with elevated CEA levels was significantly lower than those with normal CEA levels (76.5% vs 95.8%, P = 0.007). Multivariate analysis demonstrated that CEA level (OR = 8.013, 95% CI 1.573-40.817, P = 0.012), expression of Ki67 (OR = 3.298, 95% CI 0.799-13.610, P = 0.099), male gender (OR = 7.212, 95% CI 1.293-40.237, P = 0.024) and anemia (OR = 6.461, 95% CI 1.537-27.151, P = 0.011) were independent prognostic factors for the 3-year DFS. Stratified analysis revealed that elevated level of CEA combined with high expression of Ki67 was associated with a poorer prognosis (3-year DFS = 70%).
CONCLUSION: Elevated preoperative serum level of CEA and high expression of Ki67 in cancer tissue are predictors of poor prognosis for stage Ⅱa colon cancer. More intensive adjuvant treatment should be considered in patients with such high risk factors.
- Citation: Wang L, Gu J, Peng YF. Prognostic factors of stage Ⅱa colon cancer. Shijie Huaren Xiaohua Zazhi 2012; 20(36): 3816-3821
- URL: https://www.wjgnet.com/1009-3079/full/v20/i36/3816.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i36.3816
结肠癌已成为我国最常见的消化系肿瘤之一, 且其发病率呈上升趋势[1]. 临床上, 约有30%-40%的结肠癌在根治性切除后被确定为Ⅱ期(即pT3/4N0M0)[2,3]. 目前各种诊疗指南对Ⅱ期结肠癌患者是否需要接受辅助治疗尚存在争议[4-7], 仅主张对Ⅱb期具有高危因素的结肠癌患者进行辅助治疗[8,9]. Ⅱa期的结肠癌患者通常被认为预后较好、不需接受辅助治疗; 但临床发现: 约有20%-25%的Ⅱa期结肠癌患者会在术后3年内发生复发和转移[10]. 因此, 甄别Ⅱa期结肠癌的高危因素就成为一个亟待解决的临床问题[11]. 本研究采用单因素和多因素生存分析方法, 对北京大学肿瘤医院结直肠外科收治的Ⅱa期结肠癌患者的临床病理资料、血清学肿瘤标志物指标、免疫组织化学结果以及3年无病生存率(3 years disease free survival, 3yrDFS)等进行回顾性分析, 以探索Ⅱa期结肠癌的预后因素.
选取2004-01/2008-06所经治的161例Ⅱ期结肠癌中随机选取84例Ⅱa期结肠癌患者进行研究. 其中男50例, 女34例. 病变部位: 升结肠癌36例, 横结肠癌7例, 降结肠癌19例, 乙状结肠癌22例. 术后病理淋巴结检出数: 4-48(平均14.5)枚; 其中检出>12枚者59例(70.2%). 镜下见肿瘤分化不良者18例, 伴有脉管癌栓者2例. (1)入选标准: 接受了开放的结肠癌根治性切除术, 手术符合目前所提倡的全结肠系膜切除标准[12]; 术后病理分期为pT3N0M0; 有完整的临床、病理和随访资料, 且免疫组织化学检查项目均一性较好; 患者术后未进行辅助放化疗; (2)排除标准: 罹患其他恶性肿瘤的病史; 遗传性结肠癌; 根据临床分期和术中所见存在合并同时性转移的结肠癌; 合并炎症性肠病的结肠癌. 鼠抗人Ki67单克隆抗体、鼠抗人P53单克隆抗体购自德国Dako公司; SP免疫组织化学试剂盒和DAB显色试剂盒均购自北京中杉生物技术有限公司.
1.2.1 血清学CEA检测: 入选患者均在手术前1 wk内采外周静脉血, 送检进行血清CEA水平的定量测试. 所有样本均在北京大学肿瘤医院检验科进行检验, CEA滴度的判定使用Elecsys CEA reagent kit for Modular Analytics E170/cobas e 601/cobas e 602试剂盒(Roche, Germany), 以5 ng/mL作为临界值判断CEA是否升高.
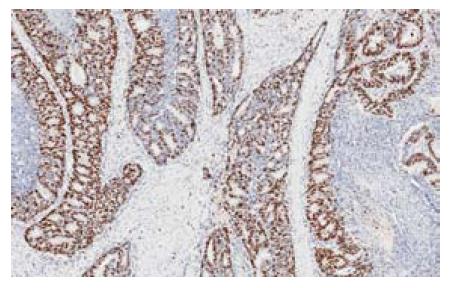
1.2.2 免疫组织化学: 北京肿瘤医院病理科对于结肠癌手术切除标本行免疫组织化学检测, 检测包括Ki67和P53等指标, 实验按产品说明书操作. 切片常规脱蜡至水, Ki67、P53采用高压热修复. 30 mL/L H2O2孵育10 min, 蒸馏水冲洗, PBS浸泡5 min, 滴加正常兔血清工作液, 孵育10 min后倾去, 不洗; 滴加一抗37 ℃孵育2 h, PBS冲洗3 min×3次. 滴加二抗, 孵育15 min, PBS冲洗3 min×3次. 滴加三抗, 孵育15 min, PBS冲洗3 min×3次, DAB显色, 苏木素复染, 脱水, 透明, 封片. PBS代替一抗作阴性对照, 正常大肠黏膜上皮表达情况作阳性对照. Ki67及P53蛋白均定位于细胞核内, 呈现棕黄色或者棕褐色颗粒, 每张组织切片随机选取10个高倍视野, 计数1 000个肿瘤细胞, Ki67蛋白按阳性细胞数占细胞总数比例分为0%-20%, >20%-40%, >40%-60%, >60%-80%, 和>80% 5组, 其中阳性细胞数>40%是定义高细胞增殖指数组, 而阳性细胞数≤40%是低细胞增殖指数组[13]. P53蛋白阳性细胞数占细胞总数比例≥10%定义为P53蛋白表达异常升高, 阳性细胞数<10%是定义为表达正常[13].
1.2.3 随访: 所有患者术后均按照NCCN结肠癌治疗指南进行随访, 患者术后2年内每3 mo随访, 3-5年每6 mo随访. 检查项目包括体检、血清CEA检查、血常规及生化、胸片、腹盆腔CT或超声. 对于肿瘤局部复发或远隔转移的判断, 以影像学诊断为主.
统计学处理 采用SPSS13.0统计软件进行分析. 分类变量中率的比较采用χ2检验, 显著性水准P = 0.05. 单因素生存分析采用Kaplan-Meier曲线法(Log-rank检验), 显著性水准品P = 0.05. 多因素生存分析采用COX回归模型, 显著性水准P = 0.1.
本组失访10例, 随访74例. 随访时间: 2-77 mo(中位时间45 mo). 随访病例的3yrDFS为88.1%; 其中8例发生异时性肝转移, 2例发生异时性肺转移, 未见局部复发病例.
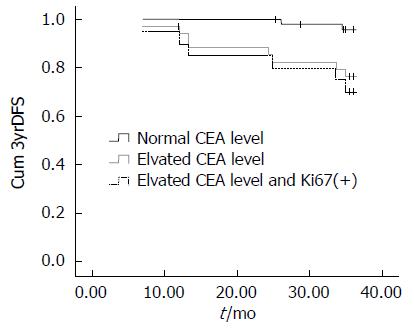
术前血清CEA水平升高者的3yrDFS为77.1%, 而CEA水平正常者的3yrDFS为95.9%, 差异显著(P = 0.007). 而高龄(年龄≥65岁)(P = 0.054)、术前贫血(P = 0.108)、肿瘤组织Ki67高表达(P = 0.104)虽无统计学差异(图1), 但显示出了影响3yrDFS的趋势(表1).
| 临床病理因素 | n | 3yrDFS(%) | P值 |
| 性别 | |||
| 男 | 50 | 84.0 | 0.144 |
| 女 | 34 | 94.1 | |
| 年龄(岁) | |||
| <65 | 42 | 95.2 | 0.054 |
| ≥65 | 42 | 81.0 | |
| 血清CEA (ng/mL) | |||
| ≤5 | 49 | 95.9 | 0.007 |
| >5 | 35 | 77.1 | |
| 血清CA199 (ng/mL) | |||
| ≤27 | 70 | 90.0 | 0.252 |
| >27 | 14 | 78.6 | |
| 血红蛋白(g/L) | |||
| <110 | 32 | 81.2 | 0.108 |
| ≥110 | 52 | 92.3 | |
| 肿瘤部位 | |||
| 右半结肠 | 43 | 86.0 | 0.521 |
| 左半结肠 | 41 | 90.2 | |
| 肿瘤长径(cm) | |||
| <5 | 38 | 89.5 | 0.702 |
| ≥5 | 46 | 87.0 | |
| 淋巴结数 | |||
| <12 | 25 | 88.0 | 0.917 |
| ≥12 | 59 | 88.1 | |
| 肿瘤分化 | |||
| 高中分化 | 66 | 86.4 | 0.335 |
| 低分化、黏液 | 18 | 94.4 | |
| 脉管癌栓 | |||
| 无 | 82 | 87.8 | N/A |
| 有 | 2 | 100.0 | |
| Ki67表达 | |||
| 低 | 46 | 93.5 | 0.104 |
| 高 | 38 | 81.6 | |
| P53表达 | |||
| 阴性 | 13 | 76.9 | 0.189 |
| 阳性 | 71 | 90.1 |
COX回归分析显示: 降低3yrDFS的独立风险因素有血清CEA水平升高(P = 0.012, OR = 8.013, 1.573-40.817), 男性(P = 0.024, OR = 7.212, 1.293-40.237), 术前贫血(P = 0.011, OR = 6.461, 1.537-27.151)以及肿瘤组织Ki67高表达(P = 0.099, OR = 3.298, 0.799-13.610). 亚组分析显示: 血清CEA升高伴Ki67高表达患者的3yrDFS(95.9%)显著低于血清CEA水平正常者(70%, P = 0.002)(图2).
临床上Ⅱ期病例约占全部结肠癌病例的40%[14,15]. 目前认为Ⅱ期结肠癌的高危因素有[8,16-18]: 血清CEA>5 ng/mL, 合并穿孔、梗阻, 急诊手术, 淋巴结<12枚, 脉管癌栓和神经侵犯等. 此外, 肿瘤浸润前缘存在肿瘤细胞出芽(tumor budding)、VEGF高表达等也与不良预后有关[19-23]. 目前普遍认为Ⅱb/c期(T4a/b, N0M0)预后较差[8,24], 因此诊疗指南普遍建议对T4期结肠癌进行辅助治疗. 但研究显示[8,14,15]: Ⅱ期结肠癌患者辅助化疗的绝对生存获益仅为2%-4%. 对于Ⅱa期结肠癌患者的风险评价仍然存在争议. 因此, 如何进一步甄别高危Ⅱ期结肠癌患者是目前研究的热点问题.
因检测手段非常成熟且性价比较高, CEA已成为结肠癌诊疗中最重要的肿瘤标志物[25]. 术前常规检测血清CEA水平被美国病理医师协会作为Ⅰ类推荐[18,26], 而美国临床肿瘤学会则建议术后应随访检测CEA水平3年以上[27]. 但术前CEA水平升高的II期结肠癌是否需要更积极的辅助治疗尚存在争议[14,15,28-30]. 多数文献[31-38]中, 术前CEA升高是结肠癌的预后不良因素, 尤其是Ⅰ-Ⅱ期的肿瘤[34,35]. 尽管90%以上的结肠癌细胞都表达CEA, 但外周血清CEA升高者仅有30%-40%, 这提示结肠癌的浸润程度和外分泌能力存在个体差异, 而这些差异也导致患者预后不同[39].
Ki67的表达水平则反映了肿瘤的原位增殖能力[13], 虽有学者[40,41]认为Ki67或P53等免疫组织化学指标与患者预后相关, 但作为预后单一因素的提示意义有限. 有学者将CEA与Ki67或P53免疫组织化学指标联合评价, 以期提高判断结肠癌预后的准确性[42,43], 但其仅测量肿瘤组织的CEA表达水平, 而忽略了血清CEA水平所提示的肿瘤浸润能力.
本研究发现: 血清CEA水平升高、肿瘤组织Ki67高表达是3yrDFS显著减低的风险因素, 合并这2种风险因素的患者, 其无病生存率(70%)显著低于Ⅱa期结肠癌的整体无病生存率(88.1%), 也差于既往文献报道的Ⅱa期结肠癌无病生存率(87.5%)[1]. 此类患者能否从更积极的辅助治疗中获益? 这还需随机对照研究提供更多证据. 本研究的多因素分析中还发现: 其他因素如男性(P = 0.024, OR = 7.212, 1.293-40.237), 术前贫血(P = 0.011, OR = 6.461, 1.537-27.151)也影响Ⅱa期结肠癌的预后, 这提示Ⅱa期结肠癌预后评价的复杂性.
目前各种诊疗指南对Ⅱ期结肠癌患者是否需要接受辅助治疗尚存在争议, 部分学者更为主张对Ⅱb期具有高危因素的结肠癌患者进行辅助治疗. Ⅱa期的结肠癌患者通常被认为预后较好、不需接受辅助治疗; 但临床发现: 约有20%-25%的Ⅱa期结肠癌患者会在术后3年内发生复发和转移. 因此, 甄别Ⅱa期结肠癌的高危因素就成为一个亟待解决的临床问题.
周晓武, 副主任医师, 中国人民解放军空军总医院普外科; 陈钟, 教授, 南通大学附属医院普外科, 南通大学肝胆外科研究所
本研究紧贴临床, 采用单因素和多因素生存分析方法, 对所收治的Ⅱa期结肠癌患者的临床病理资料、血清学肿瘤标志物指标、免疫组织化学结果以及3年无病生存率(3yrDFS)等进行回顾性分析, 研究发现: 血清CEA水平升高、肿瘤组织Ki67高表达是3yrDFS显著减低的风险因素, 合并这2种风险因素的患者, 其预后显著低于Ⅱa期结肠癌的整体预后.
本研究发现血清CEA水平升高、肿瘤组织Ki67高表达是3yrDFS显著减低的风险因素, 合并这2种风险因素的患者, 其预后显著低于Ⅱa期结肠癌的整体预后. 这有助于为临床判定Ⅱa期结肠癌的预后和指导化疗提供一个客观的参考指标.
3年无病生存率: 是指某种疾病患者经3年随访, 到随访结束时未复发转移、仍存活的病例数占观察病例的比例. 常用于评价疾病治疗的远期疗效.
本研究具有一定临床意义.
编辑: 田滢 电编:鲁亚静
| 1. | 顾 国利, 杜 长征, 薛 卫成, 顾 晋, 赵 军. 结直肠癌P27表达与Ki-67、P170、MLH1、MSH2、MSH6的关系及意义. 世界华人消化杂志. 2012;20:2157-2161. [DOI] |
| 2. | Wang W, Wang GQ, Sun XW, Chen G, Li YF, Zhang LY, Qiu HB, Huang CY, Zhan YQ, Zhou ZW. Prognostic values of chromosome 18q microsatellite alterations in stage II colonic carcinoma. World J Gastroenterol. 2010;16:6026-6034. [PubMed] |
| 3. | Mroczkowski P, Schmidt U, Sahm M, Gastinger I, Lippert H, Kube R. Prognostic factors assessed for 15,096 patients with colon cancer in stages I and II. World J Surg. 2012;36:1693-1698. [PubMed] [DOI] |
| 4. | Kosmider S, Lipton L. Adjuvant therapies for colorectal cancer. World J Gastroenterol. 2007;13:3799-3805. [PubMed] |
| 5. | Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, Tabernero J, Boni C, Bachet JB, Teixeira L. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30:3353-3360. [PubMed] [DOI] |
| 6. | Giráldez MD, Lozano JJ, Cuatrecasas M, Alonso-Espinaco V, Maurel J, Mármol M, Hörndler C, Ortego J, Alonso V, Escudero P. Gene-expression signature of tumor recurrence in patients with stage II and III colon cancer treated with 5'fluoruracil-based adjuvant chemotherapy. Int J Cancer. 2012; Jul 26. [Epub ahead of print]. [PubMed] |
| 7. | Ishiguro M, Mochizuki H, Tomita N, Shimada Y, Takahashi K, Kotake K, Watanabe M, Kanemitsu Y, Ueno H, Ishikawa T. Study protocol of the SACURA trial: a randomized phase III trial of efficacy and safety of UFT as adjuvant chemotherapy for stage II colon cancer. BMC Cancer. 2012;12:281. [PubMed] [DOI] |
| 8. | Jee SH, Moon SM, Shin US, Yang HM, Hwang DY. Effectiveness of Adjuvant Chemotherapy with 5-FU/Leucovorin and Prognosis in Stage II Colon Cancer. J Korean Soc Coloproctol. 2011;27:322-328. [PubMed] [DOI] |
| 9. | O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, Smith MA. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381-3388. [PubMed] [DOI] |
| 10. | Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427-1433. [PubMed] [DOI] |
| 11. | Tsikitis VL, Malireddy K, Green EA, Christensen B, Whelan R, Hyder J, Marcello P, Larach S, Lauter D, Sargent DJ. Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial. J Clin Oncol. 2009;27:3671-3676. [PubMed] [DOI] |
| 12. | Engel A. Complete mesocolic excision is the new kid on the block. Colorectal Dis. 2011;13:1083-1084. [PubMed] [DOI] |
| 13. | Jakob C, Liersch T, Meyer W, Becker H, Baretton GB, Aust DE. Predictive value of Ki67 and p53 in locally advanced rectal cancer: correlation with thymidylate synthase and histopathological tumor regression after neoadjuvant 5-FU-based chemoradiotherapy. World J Gastroenterol. 2008;14:1060-1066. [PubMed] [DOI] |
| 14. | Brändstedt J, Wangefjord S, Nodin B, Gaber A, Manjer J, Jirström K. Gender, anthropometric factors and risk of colorectal cancer with particular reference to tumour location and TNM stage: a cohort study. Biol Sex Differ. 2012;3:23. [PubMed] [DOI] |
| 15. | Kjaer-Frifeldt S, Hansen TF, Nielsen BS, Joergensen S, Lindebjerg J, Soerensen FB, dePont Christensen R, Jakobsen A. The prognostic importance of miR-21 in stage II colon cancer: a population-based study. Br J Cancer. 2012;107:1169-1174. [PubMed] [DOI] |
| 16. | Ogata Y, Murakami H, Sasatomi T, Ishibashi N, Mori S, Ushijima M, Akagi Y, Shirouzu K. Elevated preoperative serum carcinoembrionic antigen level may be an effective indicator for needing adjuvant chemotherapy after potentially curative resection of stage II colon cancer. J Surg Oncol. 2009;99:65-70. [PubMed] [DOI] |
| 17. | Sharif S, O'Connell MJ. Gene Signatures in Stage II Colon Cancer: A Clinical Review. Curr Colorectal Cancer Rep. 2012;8:225-231. [PubMed] [DOI] |
| 18. | Zhang NH, Li J, Li Y, Zhang XT, Liao WT, Zhang JY, Li R, Luo RC. Co-expression of CXCR4 and CD133 proteins is associated with poor prognosis in stage II-III colon cancer patients. Exp Ther Med. 2012;3:973-982. [PubMed] |
| 19. | 魏 学明, 顾 国利, 任 力, 熊 梅, 王 石林, 李 德昌. 大肠癌EGFR、HER-2、VEGF表达特点及其对分子靶向治疗的指导意义. 世界华人消化杂志. 2009;17:1836-1841. [DOI] |
| 20. | Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P, Koong AC, Kunz PA, Fisher GA, Ford JM. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128-1139. [PubMed] [DOI] |
| 21. | Chen TH, Huang CC, Yeh KT, Chang SH, Chang SW, Sung WW, Cheng YW, Lee H. Human papilloma virus 16 E6 oncoprotein associated with p53 inactivation in colorectal cancer. World J Gastroenterol. 2012;18:4051-4058. [PubMed] [DOI] |
| 22. | Abdel-Rahman WM, Ruosaari S, Knuutila S, Peltomäki P. Differential roles of EPS8 in carcinogenesis: loss of protein expression in a subset of colorectal carcinoma and adenoma. World J Gastroenterol. 2012;18:3896-3903. [PubMed] [DOI] |
| 23. | Law PC, Auyeung KK, Chan LY, Ko JK. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complement Altern Med. 2012;12:160. [PubMed] [DOI] |
| 24. | McKenzie S, Nelson R, Mailey B, Lee W, Chung V, Shibata S, Garcia-Aguilar J, Kim J. Adjuvant chemotherapy improves survival in patients with American Joint Committee on Cancer stage II colon cancer. Cancer. 2011;117:5493-5499. [PubMed] [DOI] |
| 26. | Lan YT, Yang SH, Chang SC, Liang WY, Li AF, Wang HS, Jiang JK, Chen WS, Lin TC, Lin JK. Analysis of the seventh edition of American Joint Committee on colon cancer staging. Int J Colorectal Dis. 2012;27:657-663. [PubMed] |
| 27. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [PubMed] [DOI] |
| 28. | Gulley JL, Madan RA, Tsang KY, Arlen PM, Camphausen K, Mohebtash M, Kamrava M, Schlom J, Citrin D. A pilot safety trial investigating a vector-based vaccine targeting carcinoembryonic antigen in combination with radiotherapy in patients with gastrointestinal malignancies metastatic to the liver. Expert Opin Biol Ther. 2011;11:1409-1418. [PubMed] [DOI] |
| 29. | Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, Zeh H, Bartels CJ, Lee KK, Bartlett DL. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103:689-697. [PubMed] [DOI] |
| 30. | Bacolod MD, Barany F. Molecular profiling of colon tumors: the search for clinically relevant biomarkers of progression, prognosis, therapeutics, and predisposition. Ann Surg Oncol. 2011;18:3694-3700. [PubMed] [DOI] |
| 31. | Lesterhuis WJ, de Vries IJ, Aarntzen EA, de Boer A, Scharenborg NM, van de Rakt M, van Spronsen DJ, Preijers FW, Figdor CG, Adema GJ. A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients. Br J Cancer. 2010;103:1415-1421. [PubMed] [DOI] |
| 32. | Machado NO, Chopra PJ, Al Hamdani A. Pancreatic metastasis from colon carcinoma nine years after a hemicolectomy managed by distal pancreatectomy. A review of the literature regarding the role and outcome of pancreatic resection for colorectal metastasis. JOP. 2010;11:377-381. [PubMed] |
| 33. | Aldulaymi B, Christensen IJ, Sölétormos G, Jess P, Nielsen SE, Brünner N, Nielsen HJ. Changes in soluble CEA and TIMP-1 levels during adjuvant chemotherapy for stage III colon cancer. Anticancer Res. 2010;30:233-237. [PubMed] |
| 34. | Uen YH, Lin SR, Wu DC, Su YC, Wu JY, Cheng TL, Chi CW, Wang JY. Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg. 2007;246:1040-1046. [PubMed] [DOI] |
| 35. | Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010;101:396-400. [PubMed] |
| 36. | Sun LC, Chu KS, Cheng SC, Lu CY, Kuo CH, Hsieh JS, Shih YL, Chang SJ, Wang JY. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. 2009;9:288. [PubMed] [DOI] |
| 37. | Tausch C, Konstantiniuk P, Kugler F, Reitsamer R, Roka S, Pöstlberger S, Haid A. Sentinel lymph node biopsy after preoperative chemotherapy for breast cancer: findings from the Austrian Sentinel Node Study Group. Ann Surg Oncol. 2008;15:3378-3383. [PubMed] [DOI] |
| 38. | Nakatani H, Kumon T, Kumon M, Hamada S, Okanoue T, Kawamura A, Nakatani K, Hiroi M, Hanazaki K. High serum levels of both carcinoembryonic antigen and carbohydrate antigen 19-9 in a patient with sigmoid colon cancer without metastasis. J Med Invest. 2012;59:280-283. [PubMed] [DOI] |
| 39. | Mourtzikou A, Stamouli M, Kroupis C, Christodoulou S, Skondra M, Kastania A, Pectasides D, Athanasas G, Dimas C. Evaluation of carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule EpCAM (GA733-2), and carbohydrate antigen 19-9 (CA 19-9) levels in colorectal cancer patients and correlation with clinicopathological characteristics. Clin Lab. 2012;58:441-448. [PubMed] |
| 40. | Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, Allegra CJ. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25:767-772. [PubMed] [DOI] |
| 41. | Li DD, Sun T, Wu XQ, Chen SP, Deng R, Jiang S, Feng GK, Pan JX, Zhang XS, Zeng YX. The inhibition of autophagy sensitises colon cancer cells with wild-type p53 but not mutant p53 to topotecan treatment. PLoS One. 2012;7:e45058. [PubMed] [DOI] |
| 42. | Fichtner I, Slisow W, Gill J, Becker M, Elbe B, Hillebrand T, Bibby M. Anticancer drug response and expression of molecular markers in early-passage xenotransplanted colon carcinomas. Eur J Cancer. 2004;40:298-307. [PubMed] [DOI] |