修回日期: 2007-07-20
接受日期: 2007-07-28
在线出版日期: 2007-07-28
目的: 探讨一氧化氮(NO)对肠上皮细胞表达紧密连接蛋白Occludin的影响, 以研究NO对肠黏膜屏障的作用机制.
方法: 将NO的供体Sin1与肠上皮细胞株Caco-2共培养24 h, 采用MTT方法观察NO对肠上皮细胞的作用, 并分别提取细胞蛋白和总RNA, 采用免疫蛋白印迹(Western blot)蛋白半定量方法和实时定量聚合酶链式反应(RQ-PCR)方法检测不同NO浓度对Caco-2细胞表达紧密连接蛋白Occludin蛋白和mRNA表达的影响.
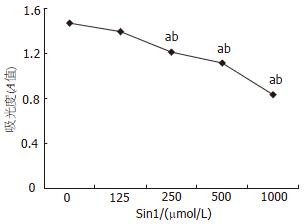
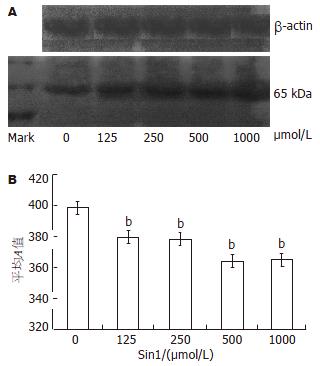
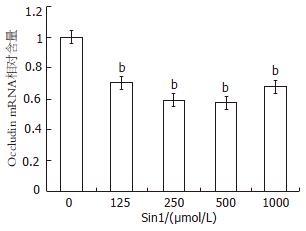
结果: 随着Sin1浓度升高(125, 250, 500和1000 μmol/L)NO对细胞的杀伤作用产生并逐渐增大, Occludin蛋白表达量和mRNA的相对表达量与无Sin1刺激时蛋白及mRNA的表达量相比明显降低(蛋白: 375±0.5, 374±0.8, 363±0.3, 363±0.7 vs 398±0.7; mRNA: 0.689±0.01, 0.578±0.09, 0.554±0.03, 0.619±0.04 vs 1, 均P<0.01).
结论: NO可直接损伤肠上皮细胞, 同时以剂量依赖形式在蛋白和分子水平影响紧密连接蛋白Occludin的表达.
引文著录: 刘冬妍, 崔巍. NO体外对肠上皮细胞表达紧密连接蛋白Occludin的影响. 世界华人消化杂志 2007; 15(21): 2295-2299
Revised: July 20, 2007
Accepted: July 28, 2007
Published online: July 28, 2007
AIM: To study the effects of NO on the intestinal mucosal barrier and on the tight junction protein occludin in intestinal epithelial cells in vitro.
METHODS: Colon cancer cell line (Caco-2 cells) was treated with Sin1, a NO donor, in a dose-dependent manner for 24 hours. The protein and total RNA of Caco-2 cells were extracted. Changes in occludin protein mRNA in Caco-2 cells stimulated by NO were determined by Western blotting and real-time quantitative pclymerase chain reaction, respectively.
RESULTS: The killing effect of NO on Caco-2 cells was dose-dependent. When treated with Sin1 at 125, 250, 500 and 1000 μmol/L doses, the levels of occludin protein (375 ± 0.5, 374 ± 0.8, 363 ± 0.3, 363 ± 0.7) and mRNA (0.689 ± 0.01, 0.578 ± 0.09, 0.554 ± 0.03, 0.619 ± 0.04) were significantly decreased compared with those in untreated Caco-2 cells (398 ± 0.7, 1, respectively, P < 0.01).
CONCLUSION: NO can directly kill intestinal epithelial cells. NO may affect protein and mRNA expression of the tight junction protein occludin in a dose-dependent manner.
- Citation: Liu DY, Cui W. Effects of NO on the tight junction protein occludin in intestinal epithelial cells in vitro. Shijie Huaren Xiaohua Zazhi 2007; 15(21): 2295-2299
- URL: https://www.wjgnet.com/1009-3079/full/v15/i21/2295.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i21.2295
Occludin是一种整体膜蛋白, 是紧密连接的重要组成部分, 对上皮细胞的紧密连接至关重要[1]. 紧密连接的建立和稳定是由生长因子、细胞因子和激素等严密调节的[2-3]. NO是细胞内和细胞间的信使, 广泛分布于消化道, 参与多种胃肠道的生理和病理过程, 如胃肠道运动、内脏的血流量调节、黏膜的保护以及炎症反应等, 是机体生理和病理生理过程中的一种重要化学介质. NO是否对紧密连接有影响, 国内报道甚少. 我们采用NO的供体Sin1与Caco-2细胞共培养, 观察NO对Occludin的影响.
Caco-2细胞株(美国ATCC公司), NO供体-Sin1、抗人Occludin抗体和b-actin(Sigma公司), RNeas Mini kit, T7体外转录试剂盒和Real time quantitative PCR试剂盒(大连宝生物工程有限公司).
参照文献[4]培养Caco-2细胞, 即应用含有200 mL/L胎牛血清, 10 g/L非必需氨基酸, 10 g/L谷氨酰胺, 1 g/L的丙酮酸钠, 青霉素-链霉素双抗液和用NaHCO3调pH值的DMEM培养液将Caco-2细胞在37℃, 50 mL/L CO2条件下进行培养, 每7 d按1:2传代, 传代后7 d细胞生长达融合状态, 每次实验分为对照组(Sin1非添加组)和实验组(Sin1添加组), 实验时取5组非同代7 d生长达融合的细胞, 添加1000 μmol/L, 500 μmol/L, 250 μmol/L, 125 μmol/L, 0 μmol/L Sin1继续培养24 h后并收集Caco-2细胞提取总蛋白和总RNA, -130℃保存以待做Western blot和Real-time PCR使用.
1.2.1 MTT检测Sin1对Caco-2杀伤: 取同代细胞接种于96孔板上, 待细胞生长达融合状态时用不同浓度Sin1处理24 h后每孔加入MTT 20 μL(5 g/L), 37℃孵箱孵育4 h, 用二甲亚砜终止反应, 492 nm波长酶标仪检测.
1.2.2 Western blot检测Sin1对Caco-2表达Occludin蛋白影响: 6孔板培养细胞达融合状态后, 加入Sin1(浓度为1000 μmol/L, 500 μmol/L, 250 μmol/L, 125 μmol/L, 0 μmol/L)培养24 h, 加入蛋白裂解液, 提取蛋白, 分装, -130℃保存待用; 用紫外分光度计进行蛋白浓度测定, 然后经SDS-聚丙烯酰胺凝胶电泳(SDS-PAGE)、转膜、染膜, 观察结果并照相, 用天能GIS凝胶图象处理系统进行分析.
1.2.3 用Real-time PCR检测Caco-2细胞Occludin: mRNA表达 取5组非同代7 d生长达融合状态的细胞, 添加1000 μmol/L, 500 μmol/L, 250 μmol/L, 125 μmol/L, 0 μmol/L Sin1在CO2孵箱中继续培养24 h, 用大连Takara公司提供的试剂提取RNA, 提取的RNA利用凝胶电泳定量. 在体外将每个样本RNA 100 ng转录为cDNA, 然后进行定量PCR反应. Occludin的引物为: Occludin-F5'-AAGAGTTGACAGTCCCATGGCATAC-3', Occludin-R5'-ATCCACAGGCGAAGTTAATGGAAG-3'; GAPDH的引物: GAPDH-F5'- GCACCGTCAAGGCTGAGAAC-3', GAPDH-R5'-ATGGTGGTGAAGACGCCAGT-3'. 将构建的RNA标准品分别梯度稀释(1010, 109, 108, 107, 106 copies/L) 作为模板进行Real-time PCR反应, 分别制作目的基因occludin和管家基因GAPDH的标准曲线. 同时使用提取RNA样品在标准曲线上分别进行定量, PCR反应条件为95℃ 10 s, 然后95℃ 5 s和60℃ 20 s循环45次, 最后经60℃ 1 min和95℃ 5 s. 按下列公式进行相对表达量分析: Occludin mRNA的相对表达量 = occludin基因拷贝数/GAPDH基因拷贝数, 校正结果以0 μmol/L为1, 其余组与之比较.
Sin1浓度为250 μmol/L, 500 μmol/L, 1000 μmol/L时对细胞有杀伤作用, 125 μmol/L无损害作用(图1).
利用Western blot方法进行蛋白的半定量分析. Occludin的分子量为65 kDa, 结果表明在65 kDa位置有明显条带, 加入Sin1后蛋白表达量明显下降, Sin1浓度为125 μmol/L, 250 μmol/L, 500 μmol/L, 1000 μmol/L时Occludin蛋白表达量分别为375±0.5, 374±0.8, 363±0.3, 363±0.7, 与无Sin1刺激Caco-2的Occludin蛋白表达量398±0.7相比明显降低(P<0.01, 图2).
应检测的mRNA以0 μmol/L Sin1时Occludin mRNA的相对表达量设为1, 通过管家基因GAPDH的校正得出Sin1浓度为125 μmol/L, 250 μmol/L, 500 μmol/L, 1000 μmol/L时Occludin mRNA的相对表达量分别为0.689±0.01, 0.578±0.09, 0.554±0.03, 0.619±0.04, Sin1浓度为125 μmol/L, 250 μmol/L, 500 μmol/L, 1000 μmol/L刺激Caco-2表达Occludin的mRNA相对含量与无Sin1刺激Caco-2表达Occludin的mRNA相对含量相比明显下降(P<0.01), 在500 μmol/L达最低, 1000 μmol/L轻微升高接近125 μmol/L(图3).
单层Caco-2细胞类似正常人肠上皮细胞, 在形态学上与人体肠上皮细胞相同, 并可分泌与人体相同的酶类、转化因子等[5], 此细胞来源于人体结肠癌细胞株, 在生长至融合后有柱状突起, 类似于小肠微绒毛. 向培养液一侧形成刷状缘并有分化良好的紧密连接, 是目前很好的体外肠屏障模型[6], 特别是在紧密连接蛋白表达研究中得到广泛应用[7-9]. 紧密连接是上皮屏障的一种重要的因子, 对相邻细胞的相互连接中起重要的作用. 他维持着细胞顶部和嗜碱性部位的分离, 是上皮渗漏的调节屏障[10]. 作为转膜蛋白的Occludin是紧密连接的重要组成部分, 他是分子质量为65 kDa的跨膜蛋白. NO由3型一氧化氮合酶(NO synthase, NOS)催化L-精氨酸合成, 他是一种多效性生物气体分子, 可参与调节血管舒张、突触传递、巨噬细胞的杀伤活性和免疫反应等, 是机体生理和病理生理过程中的一种重要化学介质[11]. 内生的NO能通过调节小肠的运动而调节肠道营养的运转[12], 他是胃肠道主要的非肾上腺素能-非胆碱能的神经递质[13], 他参与紧密连结蛋白包括Occludin的表达[14], 调节紧密连结的关和开[15], 他是紧密连结的重要调节因子[16]. 此外NO还是肠道黏膜防御的重要因子[17], 他调节黏膜免疫细胞的活性; 减少白细胞与内皮的相互黏附; 并调整黏膜血流; 减少上皮的渗漏; 刺激黏液的产生和碳酸氢盐的分泌[18-19]; 他还通过细胞毒特性进行黏膜防御[20], 由实验证实长期少量NO注射可减少实验性结肠炎症并加速其治愈[21]. 尽管在正常情况下NO对黏膜有防御作用, 但有研究显示NO对组织损伤起作用[22], 更有研究证明NOS抑制剂减弱结肠损伤和炎症反应[23-25], 此外NO还通过抑制CAMP依赖的CFTR(一种上皮细胞离子通道和氯分泌调节蛋白)抑制氯的分泌[26], 他能使细胞间紧密连接变得松弛而导致肠黏膜通透性增高, 能直接作用于肠黏膜, 导致高通透和细菌易位[27], 而且通过形成过氧化硝酸盐来损伤肠黏膜上皮[28]. 在体外NO作用于肠上皮细胞可导致钠、钾ATP酶抑制[29].
Sin1是NO的供体, 他可产生NO和等量的超氧化物, 用Sin1与细胞共培养24 h可引起大约50%细胞死亡[30]. 我们用Sin1刺激Caco-2, 观察NO对肠上皮表达Occludin的影响. MTT结果显示Sin1随浓度的加大对Caco-2细胞有杀伤作用, 这证明NO可直接作用于肠上皮细胞, 引起肠上皮细胞的损伤. NO的生物学作用除作为生物信使外, 还具有细胞毒作用, NO可作用于巯基使能量代谢或与抗氧化有关的酶失活, 并可直接损伤细胞DNA. 国外有学者利用NO另一供体-DETA-NONOate处理Caco-2发现, NO从分子和蛋白水平降低了紧密连接蛋白的表达[31-32]. 我们利用Western blot方法进行蛋白半定量检测发现加入Sin1后Occludin蛋白表达下降, 应用Real-time PCR检测加入Sin1后Caco-2表达Occludin mRNA水平得出加入Sin1后Occludin mRNA表达也明显减少, 这与国外学者报道相符. 由此得出NO在分子和蛋白水平影响紧密连接蛋白Occludin的表达, 并可直接杀伤肠上皮细胞, 损伤肠黏膜机械屏障.
国内报道NO对紧密连接影响甚少, 本文采用NO与Caco-2细胞共培养, 观察NO对 Occludin的影响.尽管许多国外学者在NO对紧密连接的影响上做了大量工作, 但NO影响紧密连接的机制还有待解决.
NO是细胞内和细胞间的信使, 参与多种胃肠道的生理和病理过程,本文采用NO的供体Sin1与Caco-2细胞共培养来观察NO体外对紧密蛋白的影响.
本文分别利用Western blot方法和Real-time PCR方法得出加入Sin1后Occludin蛋白和mRNA表达都明显减少,从而得出NO在分子和蛋白水平影响紧密连结蛋白Occludin的表达,并可直接杀伤场上皮细胞, 损伤肠黏膜屏障.
本文研究得出NO可直接损伤肠上皮细胞, 同时以剂量依赖形式在蛋白和分子水平影响紧密连接蛋白Occludin的表达, 方法成熟, 设计合理, 有一定的可读性.
编辑:王晓瑜 电编:张强
| 1. | Guo X, Rao JN, Liu L, Zou T, Keledjian KM, Boneva D, Marasa BS, Wang JY. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1159-G1169. [PubMed] |
| 2. | Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60:1091-1099. [PubMed] |
| 3. | Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851-G857. [PubMed] |
| 4. | Hamada T, Ikeda I, Takashima K, Kobayashi M, Kodama Y, Inoue T, Matsuoka R, Imaizumi K. Hydrolysis of micellar phosphatidylcholine accelerates cholesterol absorption in rats and Caco-2 cells. Biosci Biotechnol Biochem. 2005;69:1726-1732. [PubMed] |
| 5. | Yokomizo A, Moriwaki M. Transepithelial permeability of myricitrin and its degradation by simulated digestion in human intestinal Caco-2 cell monolayer. Biosci Biotechnol Biochem. 2005;69:1774-1776. [PubMed] |
| 6. | Cruz N, Qi L, Alvarez X, Berg RD, Deitch EA. The Caco-2 cell monolayer system as an in vitro model for studying bacterial-enterocyte interactions and bacterial translocation. J Burn Care Rehabil. 1994;15:207-212. [PubMed] |
| 7. | Sappington PL, Han X, Yang R, Delude RL, Fink MP. Ethyl pyruvate ameliorates intestinal epithelial barrier dysfunction in endotoxemic mice and immunostimulated caco-2 enterocytic monolayers. J Pharmacol Exp Ther. 2003;304:464-476. [PubMed] |
| 8. | Raschperger E, Engstrom U, Pettersson RF, Fuxe J. CLMP, a novel member of the CTX family and a new component of epithelial tight junctions. J Biol Chem. 2004;279:796-804. [PubMed] |
| 9. | Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1028-G1036. [PubMed] |
| 10. | Hollande F, Lee DJ, Choquet A, Roche S, Baldwin GS. Adherens junctions and tight junctions are regulated via different pathways by progastrin in epithelial cells. J Cell Sci. 2003;116:1187-1197. [PubMed] |
| 11. | Frank S, Kampfer H, Podda M, Kaufmann R, Pfeilschifter J. Identification of copper/zinc superoxide dismutase as a nitric oxide-regulated gene in human (HaCaT) keratinocytes: implications for keratinocyte proliferation. Biochem J. 2000;346 Pt 3:719-728. [PubMed] |
| 12. | Fraser R, Vozzo R, Di Matteo AC, Boeckxstaens G, Adachi K, Dent J, Tournadre JP. Endogenous nitric oxide modulates small intestinal nutrient transit and activity in healthy adult humans. Scand J Gastroenterol. 2005;40:1290-1295. [PubMed] |
| 13. | Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992;262:G379-G392. [PubMed] |
| 14. | Han X, Uchiyama T, Sappington PL, Yaguchi A, Yang R, Fink MP, Delude RL. NAD+ ameliorates inflammation-induced epithelial barrier dysfunction in cultured enterocytes and mouse ileal mucosa. J Pharmacol Exp Ther. 2003;307:443-449. [PubMed] |
| 15. | Lee NP, Cheng CY. Nitric oxide/nitric oxide synthase, spermatogenesis, and tight junction dynamics. Biol Reprod. 2004;70:267-276. [PubMed] |
| 16. | Lee NP, Mruk DD, Wong CH, Cheng CY. Regulation of Sertoli-germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS)/cGMP/protein kinase G (PRKG)/beta-catenin (CATNB) signaling pathway: an in vitro and in vivo study. Biol Reprod. 2005;73:458-471. [PubMed] |
| 17. | Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512-520. [PubMed] |
| 18. | Wallace JL. Cooperative modulation of gastrointestinal mucosal defence by prostaglandins and nitric oxide. Clin Invest Med. 1996;19:346-351. [PubMed] |
| 19. | Martin GR, Wallace JL. Gastrointestinal inflammation: a central component of mucosal defense and repair. Exp Biol Med (Maywood). 2006;231:130-137. [PubMed] |
| 20. | Marcinkiewicz J, Chain B, Nowak B, Grabowska A, Bryniarski K, Baran J. Antimicrobial and cytotoxic activity of hypochlorous acid: interactions with taurine and nitrite. Inflamm Res. 2000;49:280-289. [PubMed] |
| 21. | Wallace JL, Vergnolle N, Muscara MN, Asfaha S, Chapman K, McKnight W, Del Soldato P, Morelli A, Fiorucci S. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology. 1999;117:557-566. [PubMed] |
| 22. | Rachmilewitz D, Stamler JS, Karmeli F, Mullins ME, Singel DJ, Loscalzo J, Xavier RJ, Podolsky DK. Peroxynitrite-induced rat colitis--a new model of colonic inflammation. Gastroenterology. 1993;105:1681-1688. [PubMed] |
| 23. | Miller MJ, Thompson JH, Zhang XJ, Sadowska-Krowicka H, Kakkis JL, Munshi UK, Sandoval M, Rossi JL, Eloby-Childress S, Beckman JS. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995;109:1475-1483. [PubMed] |
| 24. | Hogaboam CM, Jacobson K, Collins SM, Blennerhassett MG. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am J Physiol. 1995;268:G673-G684. [PubMed] |
| 25. | Rachmilewitz D, Karmeli F, Okon E, Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247-255. [PubMed] |
| 26. | Skinn AC, MacNaughton WK. Nitric oxide inhibits cAMP-dependent CFTR trafficking in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G739-G744. [PubMed] |
| 27. | Xu DZ, Lu Q, Deitch EA. Nitric oxide directly impairs intestinal barrier function. Shock. 2002;17:139-145. [PubMed] |
| 28. | Nadler EP, Upperman JS, Dickinson EC, Ford HR. Nitric oxide and intestinal barrier failure. Semin Pediatr Surg. 1999;8:148-154. [PubMed] |
| 29. | Suzuki Y, Lu Q, Xu DZ, Szabo C, Hasko G, Deitch EA. Na+,K+-ATPase activity is inhibited in cultured intestinal epithelial cells by endotoxin or nitric oxide. Int J Mol Med. 2005;15:871-877. [PubMed] |
| 30. | Meij JT, Haselton CL, Hillman KL, Muralikrishnan D, Ebadi M, Yu L. Differential mechanisms of nitric oxide- and peroxynitrite-induced cell death. Mol Pharmacol. 2004;66:1043-1053. [PubMed] |