修回日期: 2007-03-01
接受日期: 2007-03-23
在线出版日期: 2007-06-28
目的: 构建在胃壁细胞中特异性表达SV40T抗原的真核表达载体并进行鉴定.
方法: 采用酚-氯仿法从昆明小鼠肝细胞中提取基因组DNA, 聚合酶链式反应(PCR)扩增H+/K+ATPase β亚基启动子, 产物命名为HK. 将PCR产物纯化回收后与pMT18-T载体相连, 并将其克隆至真核表达载体pcDNA3.1(-), 构建pcDNA3.1(-)/HK; 从含SV40T基因片段的质粒pLITAg中酶切回收SV40T基因, 与pcDNA3.1(-)/HK相连, 构建胃壁细胞特异性表达载体pcDNA3.1(-)/HKSV, 并测序鉴定.
结果: pcDNA3.1(-)/HKSV用XbaⅠ、BamHⅠ双酶切可得到1 kb H+/K+ATPase β亚基启动子, 2.7 kb SV40T基因与5.4 kb pcDNA3.1(-)载体3条DNA条带. 用XbaⅠ、KpnⅠ双酶切电泳, 可见到约3.7与5.4 kb的两条DNA条带; 用BamHⅠ单酶切电泳, 可见到2.7与6.4 kb的2条DNA条带; 用EcoR Ⅰ单酶切, 只见到约9.1 kb的1条DNA条带, 酶切电泳结果均与设计一致. 测序结果显示, H+/K+ATPase β亚基启动子与SV40T基因成功构建于pcDNA3.1(-)真核表达载体中.
结论: 构建在胃壁细胞中特异性表达SV40T基因的真核表达载体, 为进一步转基因小鼠及胃癌动物模型的建立提供了稳定、可靠的分子工具.
引文著录: 陈辉, 侯艺芳, 乐晓平, 金辉, 马慧洁, 张钦宪. SV40T抗原胃壁细胞特异性表达载体的构建与鉴定. 世界华人消化杂志 2007; 15(18): 2004-2008
Revised: March 1, 2007
Accepted: March 23, 2007
Published online: June 28, 2007
AIM: To construct and identify a eukaryotic specific expression vector of SV40 large T antigen in gastric parietal cell.
METHODS: Genome DNA was extracted from liver cells of Kunming mice by a phenol-chloroform method. The H+/K+ ATPase β subunit promoter gene was amplified by polymerase chain reaction (PCR) and the product was named as HK. This was then ligated with the pMT18-T vector after purification. A cloning vector of pcDNA3.1 (-)/HK was constructed by ligating the H+/K+ATPase β subunit promoter and the eukaryotic vector pcDNA3.1 (-). SV40 large T genes were digested by restricted enzymes from PLITAg recombinant and then inserted into the prokaryotic expression vector pcDNA3.1 (-)/HK. Thus, an expression vector specific for pcDNA3.1 (-)/HKSV was constructed and identified in gastric parietal cell.
RESULTS: Three DNA bands were seen when pcDNA3.1(-)/HKSV was digested by XbaⅠand BamHⅠ, which were the 1 kb H+/K+ ATPase β subunit promoter gene, the 2.7 kb SV40T gene and the 5.4 kb pcDNA3.1(-) vector. Two DNA bands, 3.7 kb and 5.4 kb were seen when pcDNA3.1(-)/HKSV was digested by XbaⅠ and KpnⅠ. Another two DNA bands, 2.7 kb and 6.4 kb, were seen when pcDNA3.1(-)/HKSV was digested by BamHⅠ. One DNA band, 9.1 kb, was seen when pcDNA3.1(-)/HKSV was digested by EcoRⅠ. All electrophoresis results were consistent with the design. The sequencing results showed that H+/K+ ATPase β subunit promoter and SV40 large T gene had been successfully cloned into pcDNA3.1(-) eukaryotic vector.
CONCLUSION: The recombinant plasmid pcDNA3.1 (-)/HKSV which is specially expressed in gastric parietal cell, is a stable and valuable molecular tool for establishing transgenic mice and animal models of gastric carcinoma.
- Citation: Chen H, Hou YF, Le XP, Jin H, Ma HJ, Zhang QX. Construction and identification of a specific expression vector for SV40 large T antigen in gastric parietal cell. Shijie Huaren Xiaohua Zazhi 2007; 15(18): 2004-2008
- URL: https://www.wjgnet.com/1009-3079/full/v15/i18/2004.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i18.2004
SV40(simian virus 40)是1960年代初发现分离的猿猴肾细胞病毒, 属乳多空病毒科多型瘤病毒属中的一种小DNA肿瘤病毒, 具有转化动物细胞和诱发肿瘤的特性. SV40病毒的细胞转化特性主要是与早期蛋白T抗原有关, 国外诸多研究证实了SV40T转基因小鼠模型的发瘤率很高, 可用于肿瘤发病机制的研究与转基因动物模型的建立, 如利用SV40T基因已建立了小鼠乳腺癌、脑脉络丛乳头状瘤、胰腺癌等转基因动物模型. 胃壁细胞是胃底腺的主要细胞类型之一, 能合成和分泌盐酸, 从而刺激胃肠道内分泌细胞和胰液的分泌. H+/K+ATP酶基因在胃壁细胞中特异性表达, 和胃酸的合成与分泌有着直接的关系. Gordon建立了在胃壁细胞中特异性表达人生长因子(hGH)、内在因子(INF)的转基因动物模型, 研究了胃黏膜上皮细胞的发生、演化过程, 构建的HKATP/SV40T胃癌转基因小鼠动物模型, 为胃癌的诊断、治疗提供了十分有用的实验材料. 但此方面的研究在国内未见报道, 我们拟构建在胃壁细胞中特异性表达SV40T基因的真核表达载体, 为转基因小鼠及胃癌动物模型的建立提供稳定、可靠的分子工具.
昆明小鼠由河南省实验动物中心提供, PLITAg由美国Jeffrey I. Gordon 惠赠, 克隆载体pcDNA3.1(-)由中南大学生殖与干细胞工程研究所刘永波博士惠赠, 载体pMD18-T由郑州大学公共卫生学院宋春花博士惠赠. 大肠杆菌DH5α为本室保存. T4 DNA连接酶、DL15000 DNA Marker、限制性内切酶BamHⅠ、EcoR Ⅰ、KpnⅠ和XbaⅠ为TaKaRa公司产品; 蛋白酶K、RNA酶A购自Sigma公司; 胰蛋白胨、酵母提取物购自Oxoid公司; 凝胶回收试剂盒购自TaKaRa公司.
采用酚-氯仿法从昆明小鼠肝细胞中提取基因组DNA, 紫外分光光度计测定DNA的浓度和纯度, 置-20℃保存备用.
1.2.1 PCR扩增: 以小鼠基因组DNA为模板, 设计引物扩增H+/K+ATPase b亚基启动子片段, P1: 5'-TCTAGAGCTCTTTCCTCTGGGTC-3'(含XbaⅠ位点), P2: 5'-CTCGGATTCGTCCTCTCCTGCTT-3'(含BamHⅠ位点). 反应条件为: 94℃预变性3 min; 94℃ 45 s, 60℃ 45 s, 72℃ 60 s, 共35个循环, 最后72℃延伸10 min, PCR产物命名为HK. 将PCR产物纯化回收后与pMT18-T载体相连, 转化感受态E.coli DH5α细胞, 从转化平板上随机挑取8个单菌落, 37℃摇菌过夜培养, 取菌液提取质粒DNA, XbaⅠ、BamHⅠ双酶切鉴定出阳性克隆, 命名为pMT/HK, 送北京三博远志生物工程公司测序.
1.2.2 构建pcDNA3.1/HK质粒: 将pMT/HK用XbaⅠ、BamHⅠ双酶切, 琼脂糖凝胶电泳, 切胶回收约1060 bp的DNA片段, 将回收产物与用XbaⅠ、BamHⅠ双酶切的pcDNA3.1(-)质粒连接, 目的片段与载体分子摩尔比为3:1, 45℃水浴5 min, 16℃连接16 h后, 按常规方法转化感受态DH5α细胞, 从转化平板上任意挑单菌落, 提取质粒DNA, 限制性内切酶消化鉴定出阳性重组克隆, 记作pcDNA3.1/HK.
1.2.3 构建pcDNA3.1/HKSV质粒: 将PLITAg(含SV40T基因片段)质粒用BamHⅠ酶切, 琼脂糖凝胶电泳, 切胶回收约2700 bp的DNA片段, 将回收产物与用BamHⅠ酶切的pcDNA3.1/HK连接, 目的片段与载体分子摩尔比为3:1, 反应体积为10 mL, 连接反应体系16℃连接过夜, 取2 mL连接产物转化至E.coli DH5α细胞中, 从转化平板上挑取单菌落, 37℃摇菌过夜培养, 取菌液提取质粒DNA, 酶切鉴定重组体, 并送北京三博远志生物工程公司测序鉴定SV40T基因插入的方向及DNA序列, 插入方向正确的重组体记为pcDNA3.1/HKSV.
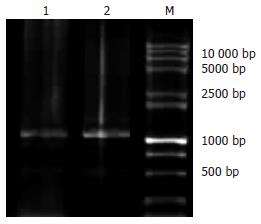
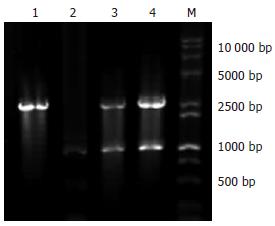
H+/K+ATPase β亚基启动子扩增产物用10 g/L琼脂糖凝胶电泳, 结果可见约1060 bp的DNA条带(图1), pMT/HK质粒用XbaⅠ、BamHⅠ双酶切电泳, 可见到1与2.6 kb的两条DNA条带, 与预期结果一致(图2).
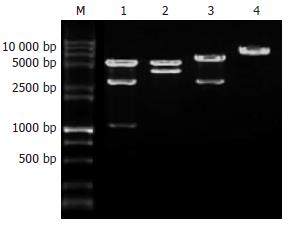
pcDNA3.1/HKSV用XbaⅠ、BamHⅠ双酶切电泳, 可见到约1, 2.7与5.4 kb的3条DNA条带; 用XbaⅠ、KpnⅠ双酶切电泳, 可见到约3.7与5.4 kb的两条DNA条带; 用BamHⅠ单酶切电泳, 可见到2.7与6.4 kb的2条DNA条带; 用EcoRⅠ单酶切, 只见到约9.1 kb的1条DNA条带, 酶切电泳结果均与设计一致(图3).
pMT/HK测序显示, PCR扩增的H+/K+ATPase β亚基启动子长度为1057 bp(-1057~-1), 测序结果与Canfield et al[1]发表序列经NCBI网站的Blast 2 sequences 对比分析, 发现在文献起始密码子上游764与765 bp之间有AC碱基的插入; -501与-634 bp之间存在碱基重复序列, 并且不同小鼠扩增产物测序以及与文献序列之间比对发现存在1-2个重复序列的不同, 推测此段序列可能为多态性位点, H+/K+ATPase β亚基启动子测序结果如下:TCTAGAGCTCTTTCCTCTGGGTCTGTTTGGTGGAACACACAGTTCTGTGTTCTCTCTAGAGCTCTTTCCTCTGGGTCTGTTTGGTGGAACACACAGTTCTG TGTTCTCACACAGTAGAGAAACAGCCTCAGCCCTCCTCCCACAGACACACGATCAGGTCATGCTGACAATCCATGTATGGTCCAGACCCTACTCATGCTTCCAG AACCTCAGAGGTGGAGTTAGGGAAATGGAGCTTTAAAAAGCTCAGTAATAACTCAGCATTTTGTTCACACATGGAGCGCCTCCATGCTCACTGAGAGACAATGG CTGTGACTCTAAGATACCTTCATGCTCTTGGTTGCCAGACCCCAGGCTTGGGTTTGCATACACAAGCTAGCTCTAACCACCCAGGTAATTTCAGGTGCCCAGAC CTCTGACTCCAGGCCTACAGGCAATTTCCAGGCCCTGAATACCTAGATCCAGGTTTGTCTAACATGCTCTCTCTCTCTCTCTCTCTCTAAATATATATATATAT ATATATATATGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTGTATATATATATATATATATATATATATATATATATATATATATACACATGGGTAT GCATATCTCCACAGGAGAATACTGAGGACAGGGCTGTGGGGCCACTCCAGGTAGTTGTAGGCACACTTTAAGCACCTTCTCCACCATCTTGAGGAGGAGGGGAG TCTCCAGGAAGCAGTTCGAGGTCCCAGGGACCTGAGTGGTGAGGTGGCAGGTCTCAGGCCTAGGGCATTTGACCTAGAAGCTGCCAGTATGTGTTTTCCCTCCT GAAGGGCAGGTGAGGCACAACCCAGAGGCTGTTCACATCAGACATGCTTCCCTTCAGCTAACATCAGGGTAGATGAAGTTGCCAGCCAAGGGCACCCCAAGGAC CAACTGACTTCTGGGACAGCGGAGGGCAGATAGCAAGCAAGCTCCAACCCTCCCTTGTGTTTGTAGAGGCGATAGTAGAGAACTGATAGCCGGTTCTGATGCCT TTGGCCTCACACAGAGGAGACTATAAGCCCTAGAGGACGCTTCCTGGGCCCAGTCCAGGCAAGCAGGAGAGGAC
注: 下划线为引物序列, 黑色区示AC碱基插入部位, 灰色区示重复碱基序列. pcDNA3.1/HKSV质粒中SV40T基因测序结果与NCBI公布的NC_001669序列完全一致.
SV40病毒属乳多空病毒科(papovaviridae)多瘤病毒属(polyomavirus), 其基因组包括5224 bp, 为双股环状DNA, 在病毒DNA复制前编码2种转化蛋白, 即大T抗原(Tag)和小T抗原(tag). 野生型SV40T基因全长2473 bp, 2个外显子连接编码序列长为2127 bp. Tag是一种磷酸化蛋白, 具有ATP酶和DNA解旋酶活性, 能使蛋白质丝氨酸、苏氨酸残基磷酸化、ADP核糖基化和乙酰基化, 另外还有活化宿主细胞核糖体基因、诱导DNA合成、修饰蛋白质合成起始因子等作用, 在细胞转化中起决定性作用, 为细胞转化所必需[2-9]. tag是非磷酸化蛋白, 对细胞转化并非必需, 但可起加强转化作用[10]. SV40T基因除用于细胞转化之外, 还发现与细胞增殖及多种肿瘤的产生相关, 并被广泛应用于转基因动物肿瘤模型的建立研究[11-20]. 如Kim et al[21]构建的共表达SV40T/抗利尿激素2转基因小鼠产生了脑瘤与淋巴瘤; Sun et al[22]将SV40T基因导入小鼠受精卵建立了前列腺癌的转基因鼠系; Nabarra et al[23]建立的在L丙酮酸激酶启动子调控下的SV40T转基因小鼠首先发生胸腺增生, 进而出现恶性胸腺瘤; Thompson et al[24]建立的CEA promotor/SV40T转基因小鼠后代在幽门处100%产生肿瘤, 37 d在小鼠胃黏膜层可观察到细胞萎缩, 50 d肿瘤可至黏膜下层, 100-130 d小鼠皆因幽门阻塞而死亡, 但由于CEA promotor/SV40T转基因小鼠基因表达的随机性, 在5000多只子鼠中仅发现一只胃癌转基因小鼠, 因此建立在胃组织特异细胞中表达的SV40T基因真核表达载体, 将为转基因小鼠及胃癌动物模型的建立提供稳定、可靠的分子工具.
胃癌是消化道恶性肿瘤中最多见的癌种, 死亡率居恶性肿瘤之首位, 建立胃癌肿瘤动物模型可为胃癌的早期诊断、治疗和发病机制的研究提供非常有用的实验动物模型[25-28]. Gordon et al研究了H+/K+ATPase β亚基在胃组织中的作用, 并建立了在胃壁细胞中特异性表达hGH, INF, SV40T的转基因动物模型[29-30], 证实了在H+/K+ATPase β亚基启动子作用下, hGH, INF, SV40T仅在小鼠胃壁细胞中表达, 在小肠、肝、幽门、贲门、大肠等处均不表达, 为胃组织特异性表达载体的构建提供了直接的理论依据. 我们成功构建了在小鼠胃壁细胞中特异性表达SV40T基因的真核表达载体, 为进一步转基因肿瘤动物的建立与研究奠定了基础.
转基因肿瘤动模型可为肿瘤发生机制的研究提供十分有用的实验动, 近年来其研究越来越受到关注. SV40(simianvirus40)属乳多空病毒科多型瘤病毒属中的一种小DNA肿瘤病毒, 具有转化动物细胞和诱发肿瘤的特性. SV40T转基因小鼠模型的发瘤效率很高, 利用SV40T基因已建立了小鼠乳腺癌、脑脉络丛乳头状瘤、胰腺癌等转基因动物模型. 建立在特定细胞中表达SV40T的转基因小鼠将可能诱发特定肿瘤的产生, 提供相应的肿瘤动物模型.
H+/K+ATP酶基因在胃壁细胞中特异性表达, 我们构建在H+/K+ATPaseβ基因启动子调控下的SV40T特异性表达载体将为转基因小鼠及胃癌动物模型的建立提供稳定、可靠的分子工具.
本文为建立转基因小鼠及胃癌动物模型而构建了定向表达载体, 为进一步研究致癌机制建立了实验基础, 意义较大.
编辑: 王晓瑜 电编:张敏
| 1. | Canfield VA, Levenson R. Structural organization and transcription of the mouse gastric H+, K(+)-ATPase beta subunit gene. Proc Natl Acad Sci U S A. 1991;88:8247-8251. [PubMed] |
| 2. | Kirchhoff C, Araki Y, Huhtaniemi I, Matusik RJ, Osterhoff C, Poutanen M, Samalecos A, Sipila P, Suzuki K, Orgebin-Crist MC. Immortalization by large T-antigen of the adult epididymal duct epithelium. Mol Cell Endocrinol. 2004;216:83-94. [PubMed] |
| 3. | Bian C, Zhao K, Tong GX, Zhu YL, Chen P. Immor-talization of human umbilical vein endothelial cells with telomerase reverse transcriptase and simian virus 40 large T antigen. J Zhejiang Univ Sci B. 2005;6:631-636. [PubMed] |
| 4. | Hofmann MC, Braydich-Stolle L, Dettin L, Johnson E, Dym M. Immortalization of mouse germ line stem cells. Stem Cells. 2005;23:200-210. [PubMed] |
| 5. | Fujii S, Maeda H, Wada N, Kano Y, Akamine A. Establishing and characterizing human periodontal ligament fibroblasts immortalized by SV40T-antigen and hTERT gene transfer. Cell Tissue Res. 2006;324:117-125. [PubMed] |
| 6. | Kowolik CM, Liang S, Yu Y, Yee JK. Cre-mediated reversible immortalization of human renal proximal tubular epithelial cells. Oncogene. 2004;23:5950-5957. [PubMed] |
| 7. | Zhang H, Tsao SW, Jin C, Strombeck B, Yuen PW, Kwong YL, Jin Y. Sequential cytogenetic and molecular cytogenetic characterization of an SV40T-immortalized nasopharyngeal cell line transformed by Epstein-Barr virus latent membrane protein-1 gene. Cancer Genet Cytogenet. 2004;150:144-152. [PubMed] |
| 8. | Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77:446-451. [PubMed] |
| 9. | Qiu HY, Fujimori Y, Nishioka K, Yamaguchi N, Hashimoto-Tamaoki T, Sugihara A, Terada N, Nagaya N, Kanda M, Kobayashi N. Postnatal neovascularization by endothelial progenitor cells immortalized with the simian virus 40T antigen gene. Int J Oncol. 2006;28:815-821. [PubMed] |
| 10. | Wen CC, Cheng SA, Hsuen SP, Huang YL, Kuo ZK, Lee HF, Kuo CH, Du JL, Wang WB. SV40 T/t-common polypeptide specifically induces apoptosis in human cancer cells that overexpress HER2/neu. Cancer Res. 2006;66:5847-5857. [PubMed] |
| 11. | Markovics JA, Carroll PA, Robles MT, Pope H, Coopersmith CM, Pipas JM. Intestinal dysplasia induced by simian virus 40 T antigen is independent of p53. J Virol. 2005;79:7492-7502. [PubMed] |
| 12. | Ishii K, Shappell SB, Matusik RJ, Hayward SW. Use of tissue recombination to predict phenotypes of transgenic mouse models of prostate carcinoma. Lab Invest. 2005;85:1086-1103. [PubMed] |
| 13. | Azzoni AR, Ribeiro SC, Monteiro GA, Prazeres DM. The impact of polyadenylation signals on plasmid nuclease-resistance and transgene expression. J Gene Med. 2007;9:392-402. [PubMed] |
| 14. | Robinson C, van Bruggen I, Segal A, Dunham M, Sherwood A, Koentgen F, Robinson BW, Lake RA. A novel SV40 TAg transgenic model of asbestos-induced mesothelioma: malignant transformation is dose dependent. Cancer Res. 2006;66:10786-10794. [PubMed] |
| 15. | McCabe MT, Low JA, Daignault S, Imperiale MJ, Wojno KJ, Day ML. Inhibition of DNA methyltrans-ferase activity prevents tumorigenesis in a mouse model of prostate cancer. Cancer Res. 2006;66:385-392. [PubMed] |
| 16. | Boissan M, Wendum D, Arnaud-Dabernat S, Munier A, Debray M, Lascu I, Daniel JY, Lacombe ML. Increased lung metastasis in transgenic NM23-Null/SV40 mice with hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:836-845. [PubMed] |
| 17. | Tani Y, Suttie A, Flake GP, Nyska A, Maronpot RR. Epithelial-stromal tumor of the seminal vesicles in the transgenic adenocarcinoma mouse prostate model. Vet Pathol. 2005;42:306-314. [PubMed] |
| 18. | Strayer DS, Cordelier P, Kondo R, Liu B, Matskevich AA, McKee HJ, Nichols CN, Mitchell CB, Geverd DA, White MK. What they are, how they work and why they do what they do? The story of SV40-derived gene therapy vectors and what they have to offer. Curr Gene Ther. 2005;5:151-165. [PubMed] |
| 19. | Konishi S, Naora H, Kimura M, Sato M, Nagasaki M, Yokoyama M, Otani H, Moritake K, Katsuki M. Expression of SV40 T antigen gene in the oligodendroglia induced primitive neuroectodermal tumor-like tumors in the mouse brain. Congenit Anom (Kyoto). 2004;44:215-224. [PubMed] |
| 20. | Delgado JP, Parouchev A, Allain JE, Pennarun G, Gauthier LR, Dutrillaux AM, Dutrillaux B, Di Santo J, Capron F, Boussin FD. Long-term controlled immortalization of a primate hepatic progenitor cell line after Simian virus 40 T-Antigen gene transfer. Oncogene. 2005;24:541-551. [PubMed] |
| 21. | Kim SH, Kim MO, Lee SR, Kim KS, Lee TH, Lee HT, Ha JH, Kim TY, Ryoo ZY. Characterization of a brain tumor cell line established from transgenic mice expressing the vasopressin SV-40 T antigen. Exp Mol Med. 2006;38:196-202. [PubMed] |
| 22. | Sun Q, Feng J, Wei XL, Zhang R, Dong SZ, Shen Q, Dong J, Li HD, Hu YH. Generation and characterization of a transgenic mouse model for pancreatic cancer. World J Gastroenterol. 2006;12:2785-2788. [PubMed] |
| 23. | Nabarra B, Pontoux C, Godard C, Osborne-Pellegrin M, Ezine S. Neoplastic transformation and angiogenesis in the thymus of transgenic mice expressing SV40 T and t antigen under an L-pyruvate kinase promoter (SV12 mice). Int J Exp Pathol. 2005;86:397-413. [PubMed] |
| 24. | Thompson J, Epting T, Schwarzkopf G, Singhofen A, Eades-Perner AM, van Der Putten H, Zimmermann W. A transgenic mouse line that develops early-onset invasive gastric carcinoma provides a model for carcinoembryonic antigen-targeted tumor therapy. Int J Cancer. 2000;86:863-869. [PubMed] |
| 25. | Takaishi S, Wang TC. Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci. 2007;98:284-293. [PubMed] |
| 26. | Taketo MM. Mouse models of gastrointestinal tumors. Cancer Sci. 2006;97:355-361. [PubMed] |
| 27. | Pritchard DM, Przemeck SM. Review article: How useful are the rodent animal models of gastric adenocarcinoma? Aliment Pharmacol Ther. 2004;19:841-859. [PubMed] |
| 28. | Mutoh H, Sakurai S, Satoh K, Osawa H, Hakamata Y, Takeuchi T, Sugano K. Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice. Gut. 2004;53:1416-1423. [PubMed] |
| 29. | Mills JC, Andersson N, Hong CV, Stappenbeck TS, Gordon JI. Molecular characterization of mouse gastric epithelial progenitor cells. Proc Natl Acad Sci U S A. 2002;99:14819-14824. [PubMed] |
| 30. | Syder AJ, Karam SM, Mills JC, Ippolito JE, Ansari HR, Farook V, Gordon JI. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc Natl Acad Sci U S A. 2004;101:4471-4476. [PubMed] |