修回日期: 2004-11-21
接受日期: 2004-12-08
在线出版日期: 2005-02-15
目的: 探讨三羟基异黄酮诱导胃癌原代细胞发生凋亡的可能性, 揭示该凋亡发生与基因bcl-2和bax之间的关系.
方法: 在体外实验中, 采用MTT比色法测定三羟基异黄酮对胃癌原代细胞的生长抑制率; 以透射电镜和TUNEL染色法, 定性、定量地研究三羟基异黄酮与胃癌原代细胞凋亡的关系; 通过免疫组织化学法和RT-PCR法检测基因bcl-2和bax的表达.
结果: 三羟基异黄酮对胃癌原代细胞有明显抑制作用,随三羟基异黄酮浓度增加和培养时间的延长而增强; 三羟基异黄酮诱导胃癌原代细胞出现凋亡细胞形态; TUNEL染色法可见, 经三羟基异黄酮处理24, 48, 72, 96 h 后, 胃癌原代细胞凋亡数明显随时间增加(1.25±0.30%→4.97±0.80%, 18.44±1.92%, 35.18±0.35%, 43.93±1.11%, P<0.05). 免疫组织化学发现经三羟基异黄酮处理24, 48, 72, 96 h后, 胃癌原代细胞的Bcl-2蛋白阳性率减少(36.34±0.72%→21.62±0.08%, 10.60±0.49%, 7.21±0.45%, 4.54±0.36%, P<0.01), Bax蛋白阳性率增加(10.73±0.57%→20.63±0.86%, 34.3±0.81%, 45.96±0.42%, 58.61±1.46%, P<0.01).RT-PCR也发现经三羟基异黄酮处理24, 48, 72, 96 h后, 胃癌原代细胞的bcl-2 mRNA条带密度降低, bax mRNA条带密度加强.
结论: 三羟基异黄酮可能通过下调bcl-2的表达和上调bax的表达而诱导胃癌原代细胞发生凋亡.
引文著录: 周海波, 颜云, 蔡建庭, 杜勤, 陈金明. 三羟基异黄酮诱导胃癌原代细胞凋亡的分子机制. 世界华人消化杂志 2005; 13(4): 504-507
Revised: November 21, 2004
Accepted: December 8, 2004
Published online: February 15, 2005
AIM: To investigate the apoptosis in primary gastric cancer cells induced by genistein, and its relationship with bcl-2 and bax.
METHODS: MTT assay was used to determine cell growth inhibition. Transmission electron microscope and TUNEL staining were used to quantitatively and qualitatively detect apoptosis. Immunohistochemistry and RT-PCR were used to detect the expression of apoptosis-regulated genes bcl-2 and bax.
RESULTS: Genistein inhibited the growth of primary gastric cancer cells in a dose- and time-dependent manner. Genistein induced primary gastric cancer cells to undergo apoptosis with typically apoptotic characteristics. TUNEL assay showed that the percentage of apoptotic cells was increased gradually along with the time of genistein treatment(1.25±0.30%, 4.97±0.80%, 18.44±1.92%, 35.18±0.35%, and 43.93±1.11% at 0, 24, 48, 72 and 96 h after treatment, respectively, P<0.05). The percentage of bcl-2 protein positive cells was significantly reduced (36.34±0.72%, 21.62±0.08%, 10.60±0.49%,7.21±0.45%,and 4.540.36% at 0, 24, 48, 72 and 96 h after treatment, respectively, P<0.01), whereas the percentage of bax protein positive cells was markedly increased (10.73±0.57%, 20.630.86%, 34.3±0.81%, 45.96±0.42%, and 58.61±1.46%, at 0, 24, 48, 72 and 96 h after treatment, respectively, P<0.01). After exposed to 20 mmol/L genistein for 24,48,72 and 96 h, bcl-2 mRNA was decreased, while bax mRNA was increased progressively with elongation of genistein treatment time.
CONCLUSION: Genistein can induce apoptosis in primary gastric cancer, which may be mediated by down-regulating the apoptosis-regulated gene bcl-2 and up-regulating the apoptosis-regulated gene bax.
- Citation: Zhou HB, Yan Y, Cai JT, Du Q, Chen JM. Genistein induces apoptosis in human primary gastric carcinoma cells. Shijie Huaren Xiaohua Zazhi 2005; 13(4): 504-507
- URL: https://www.wjgnet.com/1009-3079/full/v13/i4/504.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i4.504
Bcl-2家族在细胞凋亡中起重要作用. Bcl-2家族包括一些含同源氨基酸序列的蛋白质, 如抗凋亡蛋白Bcl-2和Bcl-xl及促凋亡蛋白Bax和Bad. 体外实验中, Bcl-2过度表达抑制细胞凋亡, 而Bax过度表达加速细胞凋亡. 三羟基异黄酮是大豆中的主要成分. 三羟基异黄酮在受体结合和细胞培养上表现了雌激素样性质. 最近研究表明三羟基异黄酮还是一种化疗药物. 三羟基异黄酮的抗肿瘤作用可能与诱导细胞凋亡有关, 但其具体作用机制还不清楚. 我们通过透射电镜和TUNEL 染色法研究三羟基异黄酮与胃癌原代细胞凋亡的关系; 通过免疫组织化学法和RT-PCR检测凋亡相关基因bcl-2和bax的表达, 研究三羟基异黄酮诱导胃癌原代细胞凋亡发生的可能机制.
三羟基异黄酮、MTT购于Sigma化学试剂有限公司. 原位凋亡检测试剂盒、Bcl-2 mAb、Bax mAb, 均为中山生物公司产品. 用二甲基亚砜(DMSO)溶解三羟基异黄酮, 以40 mmol/L浓度冷藏. 工作浓度以细胞培养液稀释. 取手术切除的新鲜胃癌组织标本, 制备单细胞悬液, 调整单细胞悬液至活细胞浓度为5×108/L. 培养后人工纯化为胃癌细胞.
1.2.1 MTT比色法: 将胃癌原代细胞以105/孔接种于96孔板, 24 h后更换培养液, 实验组分别加入含5, 10, 20, 40 mmol/L三羟基异黄酮, 每组设3个复孔, 分别培养24, 48, 72 h后每孔加入5 g/L MTT 10 mL, 继续放入培养箱37 ℃孵育4 h后, 小心吸出上清, 加入DMSO 0.2 mL/孔, 微量振荡器振荡10 min后, 在全自动酶标仪读取试验波长570 nm的吸光度(A), 计算细胞生长抑制率=对照组A值-实验组A值/对照组A值-空白组A值×100%.
1.2.2 透射电镜: 20 mmol/L三羟基异黄酮处理的胃癌原代细胞孵育24 h后, 胰蛋白酶消化收集, 用40 g/L戊二醛固定, Epon 821浸润, 60 ℃包埋72 h, 60 nm超薄切片, 醋酸双氧铀-柠檬酸铅双重染色透射电镜观察.
1.2.3 TUNEL染色法: 原位细胞凋亡检测试剂盒检测胃癌原代细胞凋亡. 加含20 mmol/L三羟基异黄酮与细胞共育24, 48, 72, 96 h. 每个时间组均设PBS对照孔. 800 mL/L酒精固定24 h, 3 mL/L过氧化氢溶液及预冷的蛋白酶K处理细胞, 37 ℃湿盒内荧光素dUTP标记1 h, POD辣根过氧化酶结合, DAB显色, 苏木素复染. 阴性对照用去掉荧光素dUTP标记体系处理标本. 染色后细胞核呈棕褐色的细胞被判为凋亡细胞. 凋亡指数(AI) = (凋亡细胞数÷总细胞数)×100%.
1.2.4 免疫组织化学法: 免疫组织化学法利用卵白素生物素技术. 在6孔板上, 20 mmol/L三羟基异黄酮与原代细胞共育24, 48, 72, 96 h. 设对照孔. 丙酮固定. PBS洗涤后, 3 mL/L过氧化氢溶液室温处理5 min, 与1:300抗-Bcl-2或抗-Bax 4 ℃过夜, PBS冲洗, 加二抗即生物素标记的抗鼠IgG室温孵育1 h.PBS冲洗, 加ABC复合剂孵育10 min.DAB显色10 min, 苏木素复染, 光学显微镜下观察棕褐色颗粒. 阴性对照用去掉一抗体系处理标本. 阳性率(PR) = (阳性细胞数/总细胞数)×100%.
1.2.5 RT-PCR检测基因bcl-2和bax的mRNA表达: 20 mmol/L三羟基异黄酮与原代细胞共育24, 48, 72, 96 h. 设对照组. 收集细胞提取总RNA.β-actin引物序列为正义链5'GTGGGGCGCCCCAGGCACCA3', 反义链5'CTCCTTAATGTCACGCACGATTTC3', 扩增片段500 bp; bcl-2引物序列为正义链5'GGAAATATGGCGCACGCT3', 反义链5'TCACTTGTGGCCCAGAT3', 扩增片段716 bp; bax引物序列为正义链 5'CCAGCTCTGAGCAGATCAT3', 反义链5'TATCAGCCCATCTTCTTCC 3', 扩增片段508 bp.PCR反应在25 mL反应体系中进行. bcl-2和β-actin的热循环条件: 94 ℃预变性7 min, 然后94 ℃ 1 min, 72 ℃ 1 min, 30个循环后, 72 ℃再延伸7 min; bax的热循环条件: 94 ℃ 1 min, 60 ℃ 45 s, 72 ℃ 45 s, 35个循环. 取 PCR扩增产物10 mL, 经15 g/L琼脂糖凝胶电泳. 紫外透射分析仪下观察.
统计学方法 配对Student t法. P<0.05具有显著性差异.
5, 10, 20, 40 mmol/L三羟基异黄酮处理原代细胞24, 48, 72 h 后, 细胞死亡随三羟基异黄酮浓度增加和培养时间的延长而增加(表1).
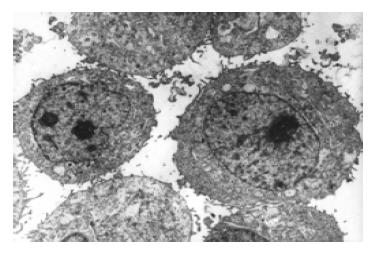
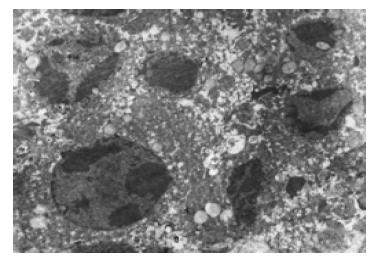
经三羟基异黄酮(20 mmol/L)处理24 h 后, 部分胃癌原代细胞出现凋亡细胞形态, 如细胞核染色质致密浓缩、聚集于核膜呈新月形小体、核碎裂(图1-2).
棕褐色染色颗粒定位于细胞核内. 经三羟基异黄酮(20 mmol/L)处理24、48、72、96 h后胃癌原代细胞凋亡指数随时间明显增加(1.25±0.30%→4.97±0.80%, 18.44±1.92%, 35.18±0.35%, 43.93±1.11%, P<0.05).
Bcl-2蛋白棕褐色染色颗粒定位于细胞质内, 经三羟基异黄酮(20 mmol/L)处理24, 48, 72, 96 h后胃癌原代细胞Bcl-2蛋白阳性率随时间明显减少(36.34±0.72%→21.62±0.08%, 10.60±0.49%, 7.21±0.45%, 4.54±0.36%, P<0.01), 表明三羟基异黄酮可下调Bcl-2 的表达. Bax蛋白棕褐色染色颗粒定位于细胞质内, 经三羟基异黄酮(20 mmol/L)处理24, 48, 72, 96 h后胃癌原代细胞Bax蛋白阳性率随时间明显增加(10.73±0.57%→20.63±0.86%, 34.3±0.81%, 45.96±0.42%, 58.61±1.46%, P<0.01), 表明三羟基异黄酮可上调Bax的表达.
三羟基异黄酮(20 mmol/L)处理24, 48, 72, 96 h后, 经RT-PCR检测发现胃癌原代细胞bcl-2和bax的mRNA表达阳性, 并显示bcl-2 mRNA条带强度随时间的延长而递减, bax mRNA条带强度随时间的延长而增强, 表明三羟基异黄酮可下调bcl-2 mRNA表达, 上调 bax mRNA表达.
三羟基异黄酮是大豆中的主要成分, 具有一个芳香族A环, 环上第2位是氧原子11.5 A, 其分子质量与雌激素类似. 三羟基异黄酮在受体结合和细胞培养上表现了雌激素样性质[1-7]. 三羟基异黄酮可抑制拓扑异构酶、血小板活化因子和表皮生长因子诱导的c-fos表达, 还可抑制甘油二酯合成和酪氨酸激酶[8-9]. 三羟基异黄酮可抑制微粒体脂质过氧化和血管生成[10-11]. 三羟基异黄酮具有抗氧化和诱导多种细胞分化作用[12]. 最近研究表明, 三羟基异黄酮还是一种化疗药物, 三羟基异黄酮的抗肿瘤作用可能与诱导细胞凋亡有关[13-22], 但其具体作用机制还不清楚.
Bcl-2家族在细胞凋亡中起重要作用. Bcl-2家族包括一些含同源氨基酸序列的蛋白质, 如抗凋亡蛋白Bcl-2和Bcl-xl及促凋亡蛋白Bax和Bad[23-26].Bax过度表达加速细胞凋亡, 而Bcl-2过度表达可抑制bax的促凋亡功能[27-36]. 因此, Bcl-2与Bax的比值是决定细胞是否凋亡的关键因素[37].
在体外实验中, MTT结果表明三羟基异黄酮对胃癌原代细胞有明显的抑制作用, 肿瘤细胞的死亡有浓度和时间依赖性. TUNEL染色法表明经20 mmol/L三羟基异黄酮处理后, 胃癌原代细胞凋亡指数随时间明显增加(P<0.05). 免疫组织化学法发现经20mmol/L三羟基异黄酮处理后, 胃癌原代细胞Bcl-2蛋白阳性率随时间明显减少(P<0.01), 胃癌原代细胞Bax蛋白阳性率随时间明显增加(P<0.01).RT-PCR发现经20 mmol/L三羟基异黄酮处理后, 胃癌原代细胞的bcl-2 mRNA条带强度递减; 而bax mRNA条带强度递增. 表明三羟基异黄酮可下调bcl-2的表达和上调bax的表达, 使bcl-2/bax比值下降, 从而导致胃癌原代细胞凋亡.
我们研究证实, 三羟基异黄酮可下调胃癌原代细胞bcl-2基因的表达而上调bax基因的表达, 从而诱导胃癌原代细胞凋亡. 有关三羟基异黄酮诱导胃癌原代细胞凋亡的确切机制, 有待进一步探讨.
编辑: 潘伯荣 审读:张海宁
| 1. | Valachovicova T, Slivova V, Sliva D. Cellular and physiological effects of soy flavonoids. Mini Rev Med Chem. 2004;4:881-887. [PubMed] [DOI] |
| 2. | Yu Z, Li W, Liu F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004;215:159-166. [PubMed] [DOI] |
| 3. | Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs. 2004;13:1327-1338. [PubMed] [DOI] |
| 4. | Sarkar FH, Li Y. The role of isoflavones in cancer chemopre-vention. Front Biosci. 2004;9:2714-2724. [PubMed] [DOI] |
| 5. | Chen AC, Berhow MA, Tappenden KA, Donovan SM. Genistein Inhibits Intestinal Cell Proliferation in Piglets. Pediatr Res. 2005;57:192-200. [PubMed] [DOI] |
| 6. | Gercel-Taylor C, Feitelson AK, Taylor DD. Inhibitory effect of genistein and daidzein on ovarian cancer cell growth. Anticancer Res. 2004;24:795-800. [PubMed] |
| 7. | Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744-757. [PubMed] [DOI] |
| 8. | Bayazit V. Cytotoxic effects of some animal and vegetable extracts and some chemicals on liver and colon carcinomaand myosarcoma. Saudi Med J. 2004;25:156-163. [PubMed] |
| 9. | Morris SM, Akerman GS, Warbritton AR, Patton RE, Doerge DR, Ding X, Chen JJ. Effect of dietary genistein on cellreplication indices in C57BL6 mice. Cancer Lett. 2003;195:139-145. [PubMed] [DOI] |
| 10. | Ye F, Wu J, Dunn T, Yi J, Tong X, Zhang D. Inhibition of cyclooxygenase-2 activity in head and neck cancer cellsby genistein. Cancer Lett. 2004;211:39-46. [PubMed] [DOI] |
| 11. | Sasamura H, Takahashi A, Yuan J, Kitamura H, Masumori N, Miyao N, Itoh N, Tsukamoto T. Antiproliferative andantiangiogenic activities of genistein in human renal cell carcinoma. Urology. 2004;64:389-393. [PubMed] [DOI] |
| 12. | Sonee M, Sum T, Wang C, Mukherjee SK. The soy isoflavone, genistein, protects human cortical neuronal cellsfrom oxidative stress. Neurotoxicology. 2004;25:885-891. [PubMed] [DOI] |
| 13. | Park OJ. Comparison of estrogen and genistein in their antigenotoxic effects, apoptosis and signal transductionprotein expression patterns. Biofactors. 2004;21:379-382. [PubMed] [DOI] |
| 14. | Su SJ, Yeh TM, Chuang WJ, Ho CL, Chang KL, Cheng HL, Liu HS, Cheng HL, Hsu PY, Chow NH. The novel targetsfor anti-angiogenesis of genistein on human cancer cells. Biochem Pharmacol. 2005;69:307-318. [PubMed] [DOI] |
| 15. | Yan SX, Ejima Y, Sasaki R, Zheng SS, Demizu Y, Soejima T, Sugimura K. Combination of genistein with ionizing radiationon androgen-independent prostate cancer cells. Asian J Androl. 2004;6:285-290. [PubMed] |
| 16. | Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cellsby the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol. 2004;25:1389-1395. [PubMed] |
| 17. | Sergeev IN. Genistein induces Ca2+ -mediated, calpain/caspase-12 dependent apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2004;321:462-467. [PubMed] [DOI] |
| 18. | Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Apoptosis of human primary gastric carcinoma cells induced by genistein. World J Gastroenterol. 2004;10:1822-1825. [PubMed] [DOI] |
| 19. | Zeng H, Chen Q, Zhao B. Genistein ameliorates beta-amyloid peptide (25-35)-induced hippocampal neuronal apoptosis.F. ree Radic Biol Med. 2004;36:180-188. [PubMed] [DOI] |
| 20. | Yu Z, Zhang L, Wu D. Genistein induced apoptosis in MCF-7 and T47D cells. Weisheng Yanjiu. 2003;32:125-127. [PubMed] |
| 21. | Li Y, Mi C. Proliferation inhibition and apoptosis onset in human ovarian carcinoma cell line SKOV3 induced by Genistein. Ai Zheng. 2003;22:586-591. [PubMed] |
| 22. | Kazi A, Daniel KG, Smith DM, Kumar NB, Dou QP. Inhibition of the proteasome activity, a novel mechanism associatedwith the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965-976. [PubMed] [DOI] |
| 23. | Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cellsby up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713-1724. [PubMed] [DOI] |
| 24. | van der Woude CJ, Jansen PL, Tiebosch AT, Beuving A, Homan M, Kleibeuker JH, Moshage H. Expression ofapoptosis-related proteins in Barrett's metaplasia-dysplasia-carcinoma sequence: A switch to a more resistantphenotype. Hum Pathol. 2002;33:686-692. [PubMed] [DOI] |
| 25. | Panaretakis T, Pokrovskaja K, Shoshan MC, Grander D. Activation of Bak, Bax and BH3-only proteins in the apoptoticresponse to doxorubicin. J Biol Chem. 2002;277:44317-44326. [PubMed] [DOI] |
| 26. | Bellosillo B, Villamor N, Lopez-Guillermo A, Marce S, Bosch F, Campo E, Montserrat E, Colomer D. Spontaneousand drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocyticleukemia. Blood. 2002;100:1810-1816. [PubMed] [DOI] |
| 27. | Matter-Reissmann UB, Forte P, Schneider MK, Filgueira L, Groscurth P, Seebach JD. Xenogeneic human NKcytotoxicity against porcine endothelial cells is perforin/granzyme B dependent and not inhibited by Bcl-2 overexpression. Xenotransplantation. 2002;9:325-337. [PubMed] [DOI] |
| 28. | Lanzi C, Cassinelli G, Cuccuru G, Supino R, Zuco V, Ferlini C, Scambia G, Zunino F. Cell cycle checkpoint efficiency andcellular response to paclitaxel in prostate cancer cells. Prostate. 2001;48:254-264. [PubMed] [DOI] |
| 29. | Mertens HJ, Heineman MJ, Evers JL. The expression of apoptosis-related proteins Bcl-2 and Ki67 in endometriumof ovulatory menstrual cycles. Gynecol Obstet Invest. 2002;53:224-230. [PubMed] [DOI] |
| 30. | Mehta U, Kang BP, Bansal G, Bansal MP. Studies of apoptosis and bcl-2 in experimental atherosclerosis in rabbit andinfluence of selenium supplementation. Gen Physiol Biophys. 2002;21:15-29. [PubMed] |
| 31. | Chang WK, Yang KD, Chuang H, Jan JT, Shaio MF. Glutamine protects activated human T cells from apoptosis byup-regulating glutathione and Bcl-2 levels. Clin Immunol. 2002;104:151-160. [PubMed] [DOI] |
| 32. | Chen GG, Lai PB, Hu X, Lam IK, Chak EC, Chun YS, Lau WY. Negative correlation between the ratio of Bax to Bcl-2 andthe size of tumor treated by culture supernatants from Kupffer cells. Clin Exp Metastasis. 2002;19:457-464. [PubMed] [DOI] |
| 33. | Usuda J, Chiu SM, Azizuddin K, Xue LY, Lam M, Nieminen AL, Oleinick NL. Promotion of photodynamic therapy-inducedapoptosis by the mitochondrial protein Smac/DIABLO: dependence on Bax. Photochem Photobiol. 2002;76:217-223. [PubMed] [DOI] |
| 34. | Sun F, Akazawa S, Sugahara K, Kamihira S, Kawasaki E, Eguchi K, Koji T. Apoptosis in normal rat embryo tissuesduring early organogenesis: the possible involvement of Bax and Bcl-2. Arch Histol Cytol. 2002;65:145-157. [PubMed] [DOI] |
| 35. | Jang M, Shin M, Shin H, Kim KH, Park HJ, Kim EH, Kim CJ. Alcohol induces apoptosis in TM3 mouse Leydig cells viabax-dependent caspase-3 activation. Eur J Pharmacol. 2002;449:39-45. [PubMed] [DOI] |
| 36. | Tilli CM, Stavast-Koey AJ, Ramaekers FC, Neumann HA. Bax expression and growth behavior of basal cell carcinomas. J Cutan Pathol. 2002;29:79-87. [PubMed] [DOI] |
| 37. | Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells viaactivation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555-561. [PubMed] [DOI] |