修回日期: 2005-11-01
接受日期: 2005-11-12
在线出版日期: 2005-12-15
目的: 对时间分辨荧光免疫法(TRFIA)与电化学发光免疫法(ECLIA)检测血清癌胚抗原CEA作比较分析, 了解两种方法的特点及这两种方法对结直肠癌检出率的影响.
方法: 分别采用TRFIA法及ECLIA法检测正常人、结直肠癌、非结直肠癌患者组共90例, 每组各30例临床送检标本血清CEA含量, 应用SPSS(Chicago, USA)软件进行统计学比较和分析.
结果: 结直肠癌组CEA值(TRFIA法: 44.12±95.27 μg/L, ECLIA法: 35.96±71.83 μg/L)明显高于正常组(TRFIA法: 1.04±0.55 μg/L, ECLIA法: 0.71±0.48 μg/L)(P<0.01). TRFIA和ECLIA的灵敏度分别为60.0%、66.7%, 特异性98.3%、100%, 准确性85.6%、88.9%, 阳性预测值94.7%、95.2%及阴性预测值83.1%、85.7%. 两种方法检测的灵敏度、特异性、准确性、阳性预测值及阴性预测值略有不同, 后者优于前者, 但两者无显著差异(P>0.05), 相关性好(r = 0.973 8).
结论: TRFIA法和ECLIA法具有良好的相关性, 并且具有灵敏度高和稳定性好等优点, 可应用于临床血清CEA检测.
引文著录: 张青云, 孙丽, 张书耕, 王琼. 时间分辨荧光免疫法与电化学发光法检测结直肠肿瘤标志 CEA 的比较. 世界华人消化杂志 2005; 13(23): 2799-2802
Revised: November 1, 2005
Accepted: November 12, 2005
Published online: December 15, 2005
AIM: To evaluate the effect of time-resolved fluorescen-ce immunoassay (TRFIA) and electro-chemilumines-cence immunoassay (ECLIA) for detecting the serum level of carcinoembryonic antigen (CEA) and their influ-ence on the positive rate of serum CEA in patients with colorectal carcinoma.
METHODS: Serum CEA levels were detected with both TRFIA and ECLIA in 90 samples from patients with colorectal carcinoma (n = 30), non-colorectal car-cinoma (n = 30), and the healthy controls (n = 30). The data were analyzed with statistical software SPSS.
RESULTS: The level of CEA was significantly higher in the colorectal cancer group (TRFIA: 44.12±95.27 μg/L, ECLIA: 35.96±71.83 μg/L) than that in the heal-thy control group (TRFIA: 1.04±0.55 μg/L, ECLIA: 0.71±0.48 μg/L) (P <0.01). The sensitivities of TRFIA and ELCIA were 60.0% and 66.7%; the specificities were 98.3% and 100%; the accuracies were 85.6% and 88.9%; the positive predictive rates were 94.7% and 95.2%; and the negative predictive rates were 83.1% and 85.7%, respectively. The results of ELCIA were better than those of the TRFIA, and they were well correlated (r = 0.973 8). There was no significant difference between the two methods (P >0.05).
CONCLUSION: Qingre Huashi recipe can increase the expression of LDL-R mRNA in the liver tissues of rat model of dampness-heat syndrome.
- Citation: Zhang QY, Sun L, Zhang SG, Wang Q. Comparison between time-resolved fluorescence immunoassay and electro-chemiluminescence immunoassay for detection of serum carcinoembryonic antigen in colorectal carcinoma. Shijie Huaren Xiaohua Zazhi 2005; 13(23): 2799-2802
- URL: https://www.wjgnet.com/1009-3079/full/v13/i23/2799.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i23.2799
癌胚抗原(carcino embryonic antigen, CEA)广泛分布于胚胎多种器官组织, 出生后血内含量很低. 他可在多种恶性肿瘤如结直肠癌、胰腺癌、胃癌、肺癌、宫颈癌等表达, 其中结直肠癌阳性率最高. 临床上CEA主要用于结直肠癌的诊断、判断肿瘤分期和病变程度、监测治疗和预报复发等[1-7], 因而通过检测血液中癌胚抗原的含量, 对动态监测跟踪结直肠癌的病情变化和观察治疗效果有重要的临床价值. 临床实验室用于检测CEA的方法主要有放射免疫分析(RIA)、酶联免疫分析(ELISA)、化学发光免疫分析(CLIA)等检测方法. 近年来发展起来的时间分辨荧光免疫分析(time-resolved fluorescence immunoassay, TRFIA)和电化学发光免疫分析(electrochemilumin escence immunoassay, ECLIA)测量技术, 具有灵敏度高、稳定性好和无放射性污染等优点[8-14], 超过了上述传统的检测方法. 而检测方法的改进是提高血清学诊断阳性率的重要途径. 因此我们采用TRFIA法与ECLIA法对比分析了临床送检的血清标本CEA的含量, 并对检测结果进行了统计分析和比较, 旨在进一步了解TRFIA法与ECLIA方法的特点及这两种方法对结直肠癌检出率的影响.
正常对照组30例为北京大学临床肿瘤学院经体检及辅助检查排除各种恶性疾病的健康人员(男4例, 女26例), 年龄23-63岁, 平均年龄35岁. 肿瘤组60例: 结直肠癌30例(男12例, 女18例), 年龄25-86岁, 平均年龄60岁; 非结直肠癌30例包括淋巴瘤、肝癌和乳癌术后(男5例, 女25例), 年龄30-74岁, 平均年龄55岁, 均为北京大学临床肿瘤学院门诊和住院患者, 肿瘤手术后标本均经病理证实.
空腹取血2 mL经4 000 r/min离心10 min, 取血清备用. 时间分辨荧光分析系统泰莱-Ⅰ及其配套试剂由广州丰华公司提供, 全自动电化学发光免疫分析系统Elecsys 2010及配套试剂由罗氏公司提供. TRFIA操作方法: 试剂及微孔反应板置室温平衡. 将微孔反应板安装到支架上, 揭去密封胶带, 洗板2次, 并在干净的吸水纸上拍干. 向微孔中加入标准品(Std)及样品(Unk)25 μL/孔. 再依次加入分析缓冲液200 μL/孔, 为避免污染应弃掉第一管. 加样时滴头应在微孔中部的上方, 避免与小孔边缘或其中的试剂接触. 微孔反应板置室温震荡温育3 h. 取下反应板, 用洗板机洗板6次(洗液加入量调节在能充满整个微孔, 并防止溢出), 并于干净的吸水纸上将微孔内残留的液体拍干, 然后加入铕标记物工作液200 μL/孔, 室温震荡温育1 h, 洗板6次, 拍干后加入增强液200 μL/孔(加入过程中避免碰到小孔边缘或其底部, 以免产生污染). 最后微孔反应板室温震荡5 min测定结果, 数据分析由其自带的LOG-LOG_B函数处理. ECLIA操作方法: 加样10 μL, 其余操作由电化学发光免疫分析仪Elecsys 2010自动完成, 两点定标. 参考数值: 95%健康人CEA≤5.0 μg/L.
统计学处理 SPSS 11.0(Chicago, USA)统计软件, 各组计量数据以均数±标准差(mean±SD)表示, 敏感性、特异性、准确性、阳性预测值及阴性预测值比较用χ2检验.
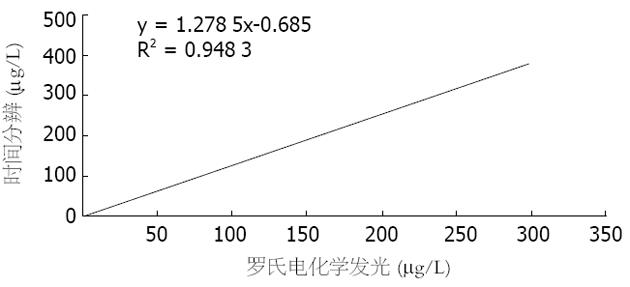
结直肠癌组CEA值(TRFIA法44.12±95.27 μg/L, ECLIA法35.96±71.83 μg/L)明显高于正常组(TRFIA法1.04±0.55 μg/L, ECLIA法0.71±0.48 μg/L), 有显著性差异(P<0.01). 以TRFIA和ECLIA的CEA≤5.0 μg/L为参考范围, 分析非结直肠癌组和结直肠癌组, 两种方法无显著差异(P>0.05). 30例正常人TRFIA和ECLIA的CEA值分别为1.04±0.55 μg/L和0.71±0.48 μg/L, 两者有显著差异(t = 3.927, P = 0.000<0.01)(表1).
CEA是一种结构复杂的可溶性糖蛋白, 分子量约为200 ku, 是从结肠癌和胎儿肠中提取的一种肿瘤相关抗原, 由内胚层细胞分化而来, 在细胞质中形成, 越过细胞膜进入体液, 故可在多种体液中检测出. 肝脏是其分解代谢的场所, 血中半衰期为2 d, 是消化系统恶性肿瘤诊断的可靠指标[15], 尤其是对结直肠癌的诊断意义最大, 胃癌次之. 人血清中CEA浓度的体外测定, 对结直肠癌等恶性肿瘤的临床诊断、疗效监测及预后评估具有重要意义[16-20].
以往对血液中肿瘤标记物CEA的检测主要依赖于放射免疫法和ELISA法, 前者由于有放射性污染, 对操作人员有危害, 且不利于环境保护. ELISA法影响因素多, 每次测定必须做标准曲线, 否则结果不稳定, 不适合单份标本的检测. 近来发展的时间分辨荧光免疫法和电化学发光免疫法均是快速、简便、无污染的检测技术. 我们运用这两种技术对30例结直肠患者、30例非结直肠患者及30例健康体检人员进行CEA检测及比较, 结果显示, 结直肠癌组CEA值(TRFIA法44.12±95.27 μg/L, ECLIA法35.96±71.83 μg/L)明显高于正常组(TRFIA法1.04±0.55 μg/L, ECLIA法0.71±0.48 μg/L), 有显著性差异(P<0.01). 本实验CEA TRFIA敏感性60.0%、特异性98.3%及阳性预测值94.7%, 与Fernandes et al[21]应用该方法测得结直肠CEA结果相近似, 分别为56%、95%、94%. 而CEA ECLIA的阳性检出率66.7%, 也与国内外文献[22,23]报道的阳性率为66.7%及68%基本相同.
TRFIA是一种新型的超微量检测技术. 其基本原理是以三价稀土离子及其螯合物作为示踪剂标记抗体, 当与相应的抗原结合时, 抗原抗体复合物在增强液中解离, 并在特定激发光激发下发出特定波长的荧光, 利用时间分辨荧光免疫测定仪测定复合物中的稀土离子发射的荧光强度, 从而确定待测抗原的量[24,25]. 该方法具有信噪比高、重复性好, 不受样品的自然荧光干扰等特点[26-28]. 而ECLIA则是采用电促化学发光, 使用非同位素金属三联吡啶钌作为标记物, 由电极发光, 在三丙胺的参与下发生的化学反应, 并以顺磁性微粒为固相载体, 从而大大提高了灵敏度, 增加了线性范围[29-31]. 目前关于这两种方法对比报道还较少, 本实验中CEA TRFIA和CEA ECLIA的灵敏度分别为60.0%、66.7%, 特异性98.3%、100%, 准确性85.6%、88.9%, 阳性预测值94.7% 、95.2%及阴性预测值83.1%、85.7%. 两种方法检测的灵敏度、特异性及准确性等略有不同, 后者优于前者, 但两者无显著差异(P>0.05), 相关性好, R = 0.973 8. 说明TRFIA和ECLIA这两种方法均适于临床CEA检测.
另外本实验中CEA ECLIA正常值明显低于CEA TRFIA, 两者有显著差异(P<0.01). 表明前者可能对低值的检测敏感性更高. 而在灵敏度、特异性、准确性等检测指标, CEA ECLIA也优于CEA TRFIA. 原因可能: (1)电化学发光免疫分析系统Elecsys 2010是全自动操作, 可保证各次实验条件一致; 而时间分辨荧光分析系统泰莱-Ⅰ则需多步手工操作, 如加样、洗涤、温育、制作标准曲线等, 人为误差较大. (2)时间分辨荧光免疫分析法CEA测定试剂盒是新研制出的国产试剂盒, 与罗氏电化学发光CEA试剂盒可能存在一定差异. 我们的综合实验结果说明TRFIA法和ECLIA法均能充分满足临床和科研试验的需求, 尤其适用于临床常规和急诊检测, 具有很好的应用前景.
电编: 张敏 编辑: 菅鑫妍 审读: 张海宁
| 1. | Engaras B, Kewenter J, Nilsson O, Wedel H, Hafstrom L. CEA, CA 50 and CA 242 in patients surviving colorectal cancer without recurrent disease. Eur J Surg Oncol. 2001;27:43-48. [PubMed] [DOI] |
| 2. | Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS. Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience. Jpn J Clin Oncol. 2000;30:12-16. [PubMed] [DOI] |
| 3. | Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338-351. [PubMed] [DOI] |
| 4. | Schiemann U, Gunther S, Gross M, Henke G, Muller-Koch Y, Konig A, Muders M, Folwaczny C, Mussack T, Holinski-Feder E. Preoperative serum levels of the carcinoembryonic antigen in hereditary non-polyposis colorectal cancer compared to levels in sporadic colorectal cancer. Cancer Detect Prev. 2005;29:356-360. [PubMed] [DOI] |
| 5. | Carpelan-Holmstrom M, Louhimo J, Stenman UH, Alfthan H, Jarvinen H, Haglund C. CEA, CA 242, CA 19-9, CA 72-4 and hCGbeta in the diagnosis of recurrent colorectal cancer. Tumour Biol. 2004;25:228-234. [PubMed] [DOI] |
| 6. | Marchena J, Acosta MA, Garcia-Anguiano F, Simpson H, Cruz F. Use of the preoperative levels of CEA in patients with colorectal cancer. Hepatogastroenterology. 2003;50:1017-1020. [PubMed] |
| 7. | Ishida H, Miwa H, Tatsuta M, Masutani S, Imamura H, Shimizu J, Ezumi K, Kato H, Kawasaki T, Furukawa H. Ki-67 and CEA expression as prognostic markers in Dukes' C colorectal cancer. Cancer Lett. 2004;207:109-115. [PubMed] [DOI] |
| 9. | Deaver DR. A new non-isotopic detection system for immun-oassays. Nature. 1995;377:758-760. [PubMed] [DOI] |
| 10. | Yan F, Zhou J, Lin J, Ju H, Hu X. Flow injection immunoassay for carcinoembryonic antigen combined with time-resolved fluorometric detection. J Immunol Methods. 2005;305:120-127. [PubMed] [DOI] |
| 11. | Yuan AS, Morris ML, Yin KC, Hsieh JY, Matuszewski BK. Development and implementation of an electrochemiluminescence immunoassay for the determination of an angiogenic pol-ypeptide in dog and rat plasma. J Pharm Biomed Anal. 2003;33:719-724. [PubMed] [DOI] |
| 12. | van Ingen HE, Chan DW, Hubl W, Miyachi H, Molina R, Pitzel L, Ruibal A, Rymer JC, Domke I. Analytical and clinical evaluation of an electrochemiluminescence immunoassay for the determination of CA 125. Clin Chem. 1998;44:2530-2536. [PubMed] |
| 13. | Wu FB, Han SQ, Xu T, He YF. Sensitive time-resolved fluoroi-mmunoassay for simultaneous detection of serum thyroid- stimulating hormone and total thyroxin with Eu and Sm as labels. Anal Biochem. 2003;314:87-96. [PubMed] [DOI] |
| 14. | Fiet J, Giton F, Auzerie J, Galons H. Development of a new sen-sitive and specific time-resolved fluoroimmunoassay (TR- FIA) of chlormadinone acetate in the serum of treated menopausal women. Steroids. 2002;67:1045-1055. [PubMed] [DOI] |
| 15. | Sturgeon C. Practice guidelines for tumor marker use in the clinic. Clin Chem. 2002;48:1151-1159. [PubMed] |
| 16. | Wiratkapun S, Kraemer M, Seow-Choen F, Ho YH, Eu KW. High preoperative serum carcinoembryonic antigen predicts metastatic recurrence in potentially curative colonic cancer: results of a five-year study. Dis Colon Rectum. 2001;44:231-235. [PubMed] [DOI] |
| 17. | Wichmann MW, Muller C, Lau-Werner U, Strauss T, Lang RA, Hornung HM, Stieber P, Schildberg FW. The role of carcinoem-bryonic antigen for the detection of recurrent disease following curative resection of large-bowel cancer. Langenbecks Arch Surg. 2000;385:271-275. [PubMed] [DOI] |
| 18. | Sorbye H, Dahl O. Transient CEA increase at start of oxalip-latin combination therapy for metastatic colorectal cancer. Acta Oncol. 2004;43:495-498. [PubMed] [DOI] |
| 19. | Chen CC, Yang SH, Lin JK, Lin TC, Chen WS, Jiang JK, Wang HS, Chang SC. Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J Surg Res. 2005;124:169-174. [PubMed] [DOI] |
| 20. | Park IJ, Kim HC, Yu CS, Yoo JH, Kim JC. Cutoff values of pre-operative s-CEA levels for predicting survivals after curative resection of colorectal cancer. J Korean Med Sci. 2005;20:624-627. [PubMed] [DOI] |
| 21. | Fernandes LC, Kim SB, Matos D. Cytokeratins and carcinoemb-ryonic antigen in diagnosis, staging and prognosis of colorectal adenocarcinoma. World J Gastroenterol. 2005;11:645-648. [PubMed] [DOI] |
| 22. | Kim SB, Fernandes LC, Saad SS, Matos D. Assessment of the value of preoperative serum levels of CA 242 and CEA in the staging and postoperative survival of colorectal adenocarcino-ma patients. Int J Biol Markers. 2003;18:182-187. [PubMed] [DOI] |
| 24. | Gaillard O, Kapel N, Galli J, Delattre J, Meillet D. Time-resolved fluorometry: principles and applications in clinical biology. Ann Biol Clin. 1994;52:751-755. [PubMed] |
| 25. | Rasi S, Suvanto E, Vilpo LM, Vilpo JA. Time-resolved fluoroi-mmunoassay of 5-methyl-2'-deoxycytidine employing europium-labeled antigen as tracer. J Immunol Methods. 1989;117:33-38. [PubMed] [DOI] |
| 26. | Matsumoto K, Yuan J, Wang G, Kimura H. Simultaneous determination of alpha-fetoprotein and carcinoembryonic antigen in human serum by time-resolved fluoroimmunoassay. Anal Biochem. 1999;276:81-87. [PubMed] [DOI] |
| 27. | Ibrahim F, Giton F, Boudou P, Villette JM, Julien R, Galons H, Fiet J. Plasma 11beta-hydroxy-4-androstene-3,17-dione: comparison of a time-resolved fluoroimmunoassay using a biotinylated tracer with a radioimmunoassay using a tritiated tracer. J Steroid Biochem Mol Biol. 2003;84:563-568. [PubMed] [DOI] |
| 28. | Allicotti G, Borras E, Pinilla C. A time-resolved fluorescence immunoassay (DELFIA) increases the sensitivity of antigen- driven cytokine detection. J Immunoassay Immunochem. 2003;24:345-358. [PubMed] [DOI] |
| 29. | Erler K. Elecsys immunoassay systems using electrochemil-uminescence detection. Wien Klin Wochenschr. 1998;110:5-10. [PubMed] |
| 30. | Obenauer-Kutner LJ, Jacobs SJ, Kolz K, Tobias LM, Bordens RW. A highly sensitive electrochemiluminescence immunoassay for interferon alfa-2b in human serum. J Immunol Methods. 1997;206:25-33. [PubMed] [DOI] |
| 31. | Guglielmo-Viret V, Attree O, Blanco-Gros V, Thullier P. Com-parison of electrochemiluminescence assay and ELISA for the detection of Clostridium botulinum type B neurotoxin. J Immunol Methods. 2005;301:164-172. [PubMed] [DOI] |