修回日期: 2005-07-01
接受日期: 2005-07-08
在线出版日期: 2005-09-15
目的: 探讨细胞凋亡及Bax, Bcl-2基因表达在重症胰腺炎(SAP)肾脏损伤中的作用.
方法: 大鼠30只随机分为假手术组(SO组, n = 15)和SAP组(n = 15), 采用50 g/L 牛磺胆酸钠逆行胆胰管注射法建立SAP模型, 建模后6, 12, 18 h测定血Cr, BUN, 光镜、电镜观察肾组织病理变化, TUNEL法测定肾脏细胞凋亡, SABC免疫组化染色法测定Bax, Bcl-2基因蛋白表达.
结果: SAP组血Cr, BUN较假手术组升高, 光电镜下见肾组织损害明显.肾小球细胞凋亡数明显高于对照组(6, 12, 18 h分别为3.2±1.1 vs 0.8±0.1, P<0.05; 3.9±1.8 vs 0.9±0.1, P<0.05; 4.8±1.7 vs 0.8±0.2, P<0.01), 肾小管细胞凋亡数明显高于对照组(6, 12, 18 h分别为9.8±1.1 vs 1.3±0.5, P<0.01; 12.6±2.3 vs 1.2±0.3, P<0.01; 15.7±2.6 vs 1.3±0.3, P<0.01), 肾小球Bax蛋白表达水平明显高于对照组(6, 12, 18 h分别为3.05±0.86 vs 1.01±0.04, P<0.05; 3.37±1.13 vs 1.06±0.05, P<0.05; 4.11±1.24 vs 1.09±0.03, P<0.05), 肾小管Bax蛋白表达水平也明显高于对照组(6, 12, 18 h分别为8.17±2.16 vs 1.53±0.07, P<0.01; 12.92±1.81 vs 1.61±0.05, P<0.01; 13.08±2.53 vs 1.59±0.05, P<0.01), 并且随病程延长而逐渐增高.Bcl-2表达主要在肾小管区, 其表达水平也高于对照组(6, 12, 18 h分别为3.38±1.07 vs 1.58±0.02, P<0.05; 4.26±1.13 vs 1.59±0.06, P<0.05; 7.21±1.04 vs 1.61±0.02, P<0.01), 肾小球区的Bcl-2表达水平也高于对照组(6, 12, 18 h分别为2.18±0.65 vs1.04±0.02; 2.42±0.63 vs 1.03±0.05, P<0.05; 2.69±0.47 vs 1.08±0.04, P<0.05), 但随病程延长而逐渐下降.
结论: SAP肾脏损伤的发病与肾脏细胞凋亡有关, 可能是通过上调Bax基因蛋白表达并下调Bcl-2基因蛋白表达而引起细胞凋亡.
引文著录: 朱明德, 方驰华, 史学深. 重症胰腺炎肾脏损伤中细胞凋亡相关基因的作用. 世界华人消化杂志 2005; 13(17): 2103-2107
Revised: July 1, 2005
Accepted: July 8, 2005
Published online: September 15, 2005
AIM: To explore the roles of renal cell apoptosis and expression of Bax and Bcl-2 in renal injury in rats with severe acute pancreatitis (SAP).
METHODS: Thirty rats were randomized into sham operation (SO, n = 15) and SAP group (n = 15). The model of SAP was established by retrograde injection of 50 g/L sodium taurocholate into the biliopancreatic duct in the rats. Serum creatinine (Cr) and blood urea nitrogen (BUN) were determined 6, 12, and 18 h after injection. The pathological changes were observed by light and electron microscopy. The apoptosis of renal cells was determined by TUNEL method, and the expression of Bax and Bcl-2 was detected by immunohistochemical staining (SABC assay).
RESULTS: As compared with those in SO group at the same phase, serum Cr and BUN in SAP group increased markedly. Significant injuries of renal tissue were observed under light and electron microscope.The apoptotic index of renal glomerular cells increased significantly 6, 12, 18 h after injection (3.2±1.1 vs 0.8±0.1, P <0.05; 3.9±1.8 vs 0.9±0.1, P <0.05; 4.8±1.7 vs 0.8±0.2, P <0.01, respectively). The apoptotic index of renal tubule cells also increased significantly (9.8±1.1 vs 1.3±0.5, P <0.01; 12.6±2.3 vs 1.2±0.3, P <0.01; 15.7±2.6 vs 1.3±0.3, P <0.01, respectively). The expression of Bax in renal glomerulus and tubule increased significantly at 6, 12, 18 h, respectively (glomerulus: 3.05±0.86 vs 1.01±0.04, P <0.05; 3.37±1.13 vs 1.06±0.05, P <0.05; 4.11±1.24 vs 1.09±0.03, P <0.05; tubule: 8.17±2.16 vs 1.53±0.07, P <0.01; 12.92±1.81 vs 1.61±0.05, P <0.01; 13.08±2.53 vs 1.59±0.05, P <0.01), and increased continuously. Bcl-2 was mainly expressed in the regions of renal tubule, and it also increased in SAP group at 6, 12, 18 h, respectively (3.38±1.07 vs 1.58±0.02, P <0.05; 4.26±1.13 vs 1.59±0.06, P <0.05; 7.21±1.04 vs 1.61±0.02, P <0.01). The level of Bcl-2 expression in renal glomerulus was higher in SAP group than that in SO group at 6, 12, 18 h, respectively (2.18±0.65 vs 1.04±0.02; 2.42±0.63 vs 1.03±0.05, P <0.05; 2.69±0.47 vs 1.08±0.04, P <0.05), but decreased with the duration of the injuries.
CONCLUSION: Renal cell apoptosis, which relates to the up-regulated expression of Bax and downed regulated expression of Bcl-2, may be involved in the pathogenesis of renal injury in patients with SAP.
- Citation: Zhu MD, Fang CH, Shi XS. Renal cells apoptosis and Bax, Bcl-2 expression in rats with severe acute pancreatitis. Shijie Huaren Xiaohua Zazhi 2005; 13(17): 2103-2107
- URL: https://www.wjgnet.com/1009-3079/full/v13/i17/2103.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i17.2103
重症急性胰腺炎(SAP)常并发多器官功能障碍, 其胰外器官损伤中肾功能障碍(胰性肾病)发生率约14-43%, 仅次于肺功能障碍, 发展至急性肾功能衰竭后死亡率高达71-84%[1].目前对其发病机制尚不完全清楚.过去认为SAP能致动物肾病与磷酶脂A2、内毒素、肿瘤坏死因子、脂质过氧化损伤等有关[2-7], 近年的研究发现, 在一些继发性肾损伤过程中可发现肾脏细胞凋亡现象[8-14].我们检测SAP肾脏损伤大鼠肾组织细胞凋亡及凋亡相关基因蛋白表达情况, 探讨细胞凋亡及其相关基因蛋白表达在SAP肾脏损伤发病过程中的作用.
SD大鼠30只, 体质量250-300 g, 由南方医科大学实验动物中心提供.随机分成2组: 假手术组. (SO组)和SAP组各15只.每组再分为6, 12, 18 h三个时间点, 每个时间点分配5只大鼠.动物术前禁食不禁水12 h, SAP组用30 g/L 戊巴比妥钠(30 mg/kg)ip麻醉, 作上腹正中切口入腹, 近肝门处暂时夹闭胆总管, 于十二指肠降部找到胰胆管开口, 在对侧肠壁上选一无血管区, 用穿刺针扎穿肠壁, 将穿刺导管从针孔送入肠腔后穿入胰胆管内1 cm, 注入50 g/L牛磺胆酸钠(10 mL/kg)制备SAP模型(注射速度0.2 mL/min), 指压穿刺点, 查无漏胆, 逐层关腹; SO组仅行胰胆管穿刺操作, 不注射药物.造模后6, 12, 18 h等各时间点处死大鼠, 心脏穿刺抽血, 快速切取左侧肾脏, 测定各项指标.
全自动生化分析仪测定血Cr, BUN.各组织标本以多聚甲醛固定, 常规石蜡包埋、HE染色, 光镜观察; 电镜标本以30 g/L 戊二醛-10 g/L 锇酸双重固定, 环氧树脂包埋, 超薄切片, 醋酸铀和柠檬酸铅电子染色, 透射电镜观察.
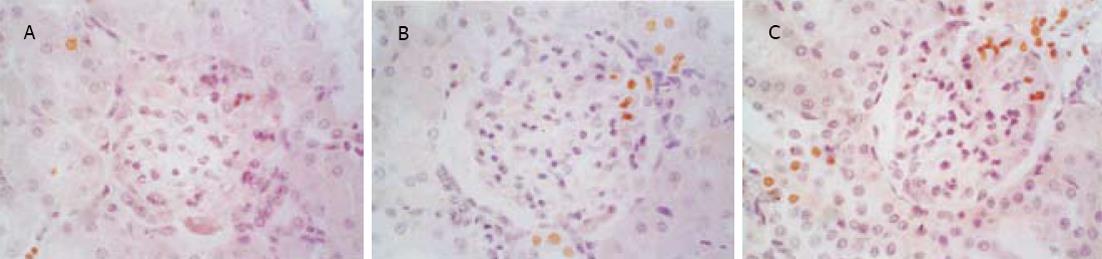
1.2.1 肾脏细胞凋亡的测定: 采用DNA末端原位标记法(TUNEL法, 试剂盒购自武汉博士德公司).切片常规脱蜡入水, 经30 mL/L H2O2处理、蛋白酶K消化后, 加入TDT和Dig.dUTP4℃过夜, 封闭后加生物素化抗地高辛抗体, 洗涤, 加SABC, DAB显色, 光镜下计算肾脏细胞凋亡指数(apoptotic index, AI).AI计算方法: 细胞核中有棕黄色颗粒者为阳性细胞, 即凋亡细胞, 每张切片均分别统计20个正切肾小球及100个正切肾小管凋亡细胞数和总细胞数, AI=凋亡细胞数/总细胞数×100%.
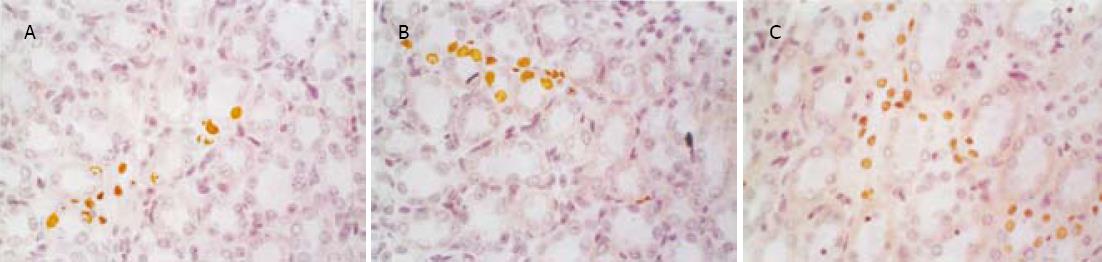
1.2.2 基因Bax, Bcl-2的测定: 采用SABC免疫组化染色法(免疫组化试剂盒购自武汉博士德公司).染色模式: 胞膜或胞质。各组切片均采用图像分析系统对免疫组化染色阳性反应产物进行定量分析.具体操作为: 每张切片分别测量10个肾小球及10个高倍视野的肾小管, 利用图像分析系统分析阳性面积和阳性区域平均灰度值, 并将阳性面积和平均灰度值按文献方法[15]换算成阳性单位(positive unit, PU), 以PU值大小代表阳性产物表达的多少.
统计学处理 采用SPSS10.0统计软件建立数据库, 两组均数比较用完全随机设计资料的方差分析, 细胞凋亡与肾功能损害的相关性用相关性分析.P<0.05为差异有显著性意义.
SO组大体未见异常; SAP组6 h时腹腔见少量渗液, 肾脏轻度水肿; 12 h时肾脏充血水肿加重, 并可见少量出血点, 腹腔渗液增多, 淡红色; 18 h时肾脏充血水肿更为明显, 可见局灶性坏死, 腹腔渗液呈血性.光镜下SO组未见异常; SAP组6 h时肾小球轻度肿胀, 肾小管肿胀较为明显, 肾间质见炎细胞浸润; 12 h时肾小球、肾小管肿胀加重, 间质炎细胞增多; 18 h时肾小球可见淤血, 肾小管上皮细胞可见坏死.电镜下SO组未见异常; SAP组6 h时肾小球毛细血管扩张, 足细胞核不规则, 线粒体凝集和肿胀, 溶酶体增多, 内质网扩张, 基膜局部增厚, 肾小管上皮细胞水肿, 表面微绒毛减少, 排列紊乱, 微绒毛末端呈杆状膨大.随病程延长, 上述病理改变加重.18 h时尚出现肾小球毛细血管淤血, 粒细胞凝集, 基膜内有纤维素沉积, 肾小管上皮细胞胞浆空泡化, 线粒体空泡变性, 表面微绒毛脱落, 部分线位体破裂, 各时段均可见细胞核固缩, 集合管内可见坏死的肾小管上皮细胞和红细胞.SAP时血Cr, BUN明显升高, 且随着病程延长而逐渐增高(表1).
SO组肾小球和肾小管仅偶见凋亡细胞, SAP组凋亡细胞明显增多, 凋亡指数升高, 并随着病程延长而逐渐增高, 肾小管凋亡现象较肾小球严重(图1, 图2, 表2).肾小管细胞凋亡与血Cr呈正相关(r = 0.812, P<0.05), 与血BUN也呈正相关(r = 0.807, P<0.05).
| 指标 | 分组 | 肾小球 | 肾小管 | ||||
| 6 h | 12 h | 18 h | 6 h | 12 h | 18 h | ||
| AI | SO | 0.80±0.10 | 0.90±0.10 | 0.80±0.20 | 1.30±0.50 | 1.20±0.30 | 1.30±0.30 |
| SAP | 3.20±1.10a | 3.90±1.80a | 4.80±1.70b | 9.80±1.10b | 12.60±2.30b | 15.7±2.6b | |
| Bax(PU) | SO | 1.01±0.04 | 1.06±0.05 | 1.09±0.03 | 1.53±0.07 | 1.61±0.05 | 1.59±0.05 |
| SAP | 3.05±0.86a | 3.37±1.13a | 4.11±1.24a | 8.17±2.16b | 12.92±1.81b | 13.08±2.53b | |
| Bcl-2(PU) | SO | 1.04±0.02 | 1.03±0.05 | 1.08±0.04 | 1.58±0.02 | 1.59±0.06 | 1.61±0.02 |
| SAP | 2.18±0.65 | 2.42±0.63a | 2.69±0.47a | 3.38±1.07a | 4.26±1.13a | 7.21±1.04b | |
| Bcl-2/Bax | SO | 1.02±0.06 | 0.98±0.05 | 0.99±0.03 | 1.03±0.05 | 0.99±0.07 | 1.01±0.05 |
| SAP | 0.72±0.01a | 0.70±0.03a | 0.65±0.03a | 0.66±0.02a | 0.62±0.03a | 0.55±0.01b | |
急性肾功能衰竭时肾小管上皮细胞出现凋亡早于肾功能的变化[16].凋亡作为细胞丢失的途径之一, 同样可造成肾小管基底膜裸露、转运功能丧失及小管液回漏等功能障碍, 成为少尿或无尿的病理生理基础, 说明凋亡在肾功能损害中发挥重要的作用[17-22].细胞凋亡也参与胰性肾病的发病过程.Takase et al[23]在诱发实验性SAP6 h后, 并发ARF的小鼠肾脏肾小管细胞出现凋亡, 用SAP小鼠产生的腹水注射到健康小鼠, 也导致肾小管细胞的凋亡; SAP时血中内毒素(ET)、肿瘤坏死因子(TNFα)等明显升高, 而经ET注射的小鼠肾脏可见到明显的细胞凋亡[24], TNFα增加了ET诱导的肾小管细胞的凋亡[25].我们发现, SAP大鼠肾脏的细胞凋亡数明显增多, 凋亡指数增高, 肾小管凋亡现象较肾小球严重, 肾脏细胞凋亡指数与肾功能的损害程度呈正相关, 说明SAP肾脏损伤至少部分是通过细胞凋亡机制引起的, 而且细胞凋亡是造成肾功能进一步恶化的重要因素之一.
细胞凋亡是受基因调控的, 在调控细胞凋亡的基因中, Bcl-2家族是最受重视者之一, 其中Bcl-2蛋白是最重要的凋亡抑制基因, 它的生理功能主要是阻遏细胞凋亡, 延长细胞寿命.Bax也是Bcl-2基因家族成员, 它的功能与Bcl-2相反, 主要是促进细胞凋亡[26-28], Bax和Bcl-2分别可以以同源二聚体形式存在, 他们之间也可形成异源二聚体, Bax-Bcl-2异源二聚体较稳定, 无诱导凋亡的作用, 而当Bax较Bcl-2绝对或相对增高, 即细胞内Bcl-2与Bax的比例(Bcl-2/Bax)下降时, Bax-Bax同源二聚体形成, 便诱导凋亡[29-30].我们的实验发现, SAP时Bax在肾小球和肾小管的表达均明显上升, 且随着病程延长而逐渐增高, Bcl-2的表达也有所上升, 但幅度相对较小, 可能是机体代偿所致, 通过Bcl-2表达的增加发挥其抗凋亡作用, 减轻细胞损伤, 总体上, Bcl-2/Bax比值逐渐下降, 导致细胞凋亡逐渐增加.我们认为SAP能致病鼠肾脏损伤, 可能是由于SAP产生的肾毒性物质上调诱导凋亡的基因蛋白表达并相对下调抑制凋亡的基因蛋白表达, 使肾小球、肾小管细胞凋亡, 引起肾小球滤过膜通透性增加, 肾小管重吸收功能障碍所致.通过降低Bax活性、提高Bcl-2的活性以干预细胞凋亡从而减轻SAP时的肾脏损伤, 无疑为胰性肾病的防治提供了新的途径.
编辑: 潘伯荣 审读: 张海宁
| 1. | Pupelis G. Renal failure in acute pancreatitis. Timing of dialysis and surgery. Przegl Lek. 2000;57:29-31. [PubMed] |
| 2. | Hietaranta AJ, Aho HJ, Gronroos JM, Hua ZY, Nevalainen TJ. Pancreatic phospholipase A2 in proximal tubules of rat kidney in experimental acute pancreatitis and after intravenous injection of the enzyme. Pancreas. 1992;7:326-333. [PubMed] [DOI] |
| 3. | Isaji S, Hayashi J, Higashiguchi T, Yokoi H, Ogura Y, Noguchi T, Kawarada Y. Effect of IS-741(a new synthetic antiinflammatory agent) on acute necrotizing pancreatitis in dogs. Significance of its inhibitory effect on cytosolic phospholipase A2. Digestion. 1999;60:47-51. [PubMed] [DOI] |
| 4. | Hughes CB, Gaber LW, Mohey el-Din AB, Grewal HP, Kotb M, Mann L, Gaber AO. Inhibition of TNF alpha improves survival in an experimental model of acute pancreatitis. Am Surg. 1996;62:8-13. [PubMed] |
| 5. | Lindsey DC, Emerson TE, Thompson TE, John AE, Duerr ML, Valdez CM, Kuo HS, Bouffard RB, Irwin RG, Canivel D. Characterization of an endotoxemic baboon model of metabolic and organ dysfunction. Circ Shock. 1991;34:298-310. [PubMed] |
| 6. | Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783-1788. [PubMed] [DOI] |
| 7. | Plusczyk T, Witzel B, Menger MD, Schilling M. ETA and ETB receptor function in pancreatitis-associated microcirculatory failure, inflammation, and parenchymal injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:145-153. [PubMed] [DOI] |
| 10. | 张 艳玲, 段 惠军, 郝 文田, 李 英敏, 史 永红, 李 英, 顾 连方. 苯那普利对糖尿病大鼠肾脏细胞凋亡及Bax和Bcl-2表达的 影响. 中华物理医学与康复杂志. 2001;23:237-239. |
| 11. | Mountz JD, Zhou T, Su X, Wu J, Cheng J. The role of programmed cell death as an emerging new concept for the pathogenesis of autoimmune diseases. Clin Immunol Immunopathol. 1996;80:S2-S14. [PubMed] [DOI] |
| 12. | Hattori T, Shindo S, Kawamuea H. Apoptosis and expression of Bax protein and Fas antigen in glomeruli of a remnant- kidney model. Nephron. 1998;79:186-191. [PubMed] [DOI] |
| 13. | Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160-1178. [PubMed] [DOI] |
| 14. | Ito K, Chen J, EI Chaar M, Stern JM, Seshan SV, Khodadadian JJ, Richardson I, Hyman MJ, Vaughan ED Jr, Poppas DP, Felsen D. Renal damage progresses despite improvement of renal function after relief of unilateral ureteral obstruction in adult rats. Am J Physiol Renal Physiol. 2004;287:F1283-F1293. [PubMed] [DOI] |
| 15. | 申 洪. 免疫组织化学显色反应强度定量方法研究. 中国组织化学与细胞化学杂志. 1995;4:89-92. |
| 17. | Hauser P, Oberbauer R. Tubular apoptosis in the pathophysiology of renal diseases. Wien Klin Wochenschr. 2002;114:671-677. [PubMed] [DOI] |
| 18. | Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol. 2003;23:511-521. [PubMed] [DOI] |
| 19. | Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002;11:301-308. [PubMed] [DOI] |
| 20. | Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608-F627. [PubMed] |
| 21. | Ortiz A, Justo P, Sanz A, Melero R, Caramelo C, Guerrero MF, Strutz F, Müller G, Barat A, Egido J. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir Ther. 2005;10:185-190. [PubMed] |
| 22. | Dieguez-Acuna FJ, Polk WW, Ellis ME, Simmonds PL, Kushleika JV, Woods JS. Nuclear factor kappaB activity determines the sensitivity of kidney epithelial cells to apoptosis: implications for mercury-induced renal failure. Toxicol Sci. 2004;82:114-123. [PubMed] [DOI] |
| 23. | Takase K, Takeyama Y, Nishikawa J, Ueda T, Hori Y, Yamamoto M, Kuroda Y. Apoptotic cell death of renal tubules in experimental severe acute pancreatitis. Surgery. 1999;125:411-420. [PubMed] [DOI] |
| 25. | Sood A, Mathew R, Trachtman H. Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to Shiga toxin. Biochem Biophys Res Commun. 2001;283:36-41. [PubMed] [DOI] |
| 26. | Eschwege P, Paradis V, Conti M, Loric S, Dumas F, Berteau P, Ahmed M, Droupy S, Charpentier B, Legrand A. Bcl-2 and Bax expression on rat ischemic kidney. Transplant Proc. 1998;30:2861-2862. [PubMed] [DOI] |
| 27. | Rawat S, Gray C, Johnson TS, Raftery AT, EI Nahas AM, Haylor J. Apoptosis and expression of BCL-2 and BAX in cyclosporine-induced experimental renal fibrosis. Transplant Proc. 2003;35:187-188. [PubMed] [DOI] |
| 28. | Yamamoto K, Tomita N, Yoshimura S, Nakagami H, Taniyama Y, Yamasaki K, Ogihara T, Morishita R. Hypoxia-induced renal epithelial cell death through caspase-dependent pathway: role of Bcl-2, Bcl-xL and Bax in tubular injury. Int J Mol Med. 2004;14:633-640. [PubMed] [DOI] |
| 29. | Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s-1700s. [PubMed] |
| 30. | Sprick MR, Walczak H. The interplay between the Bcl-2 family and death receptor-mediated apoptosis. Biochim Biophys Acta. 2004;1644:125-132. [PubMed] [DOI] |