修回日期: 2005-07-01
接受日期: 2005-07-08
在线出版日期: 2005-09-15
目的: 研究极度低氧下人胰腺癌细胞株PC-3中IAP-2表达的变化, 初步探讨其与HIF-1的相关机制.
方法: PC-3细胞株分组孵育: 常氧组, 体积分数为20 mL/L O2/50 mL/L CO2/930 mL/L N2低氧组4 h, 950 mL/L N2/50 mL/L CO2极度低氧组1、3、5 h, 950 mL/L N2/50 mL/L CO2低氧3 h后复氧, 另取一组加入300 μmol/L氯化钴常氧孵育. 用细胞免疫化学定性检测IAP-2蛋白表达; 蛋白裂解液提取胞质胞核蛋白, 用Western blot检测IAP-2蛋白水平变化, 同时对比HIF-1蛋白表达; 用RT-PCR检测IAP-2基因水平改变.
结果: 免疫细胞化学检测IAP-2蛋白在PC-3细胞中呈阳性表达, 定位于胞质. Western blot显示常氧、低氧4h可检测到IAP-2蛋白,表达无差异, 极度低氧1 h IAP-2蛋白表达明显增加(t = 3.300, P<0.05), 3、5 h IAP-2持续高表达, 各时间段无显著性差异, 复氧后恢复基线水平. 实验还显示了HIF-1和IAP-2蛋白的表达差异: 常氧组HIF-1未表达, 低氧4 h可诱发, IAP-2保持基线水平; 极度低氧HIF-1无变化, IAP-2表达明显增高; 复氧后HIF-1不表达, IAP-2恢复基线表达; 加入氯化钴后HIF-1恢复表达, IAP-2保持基线, 初步表明IAP-2蛋白表达上调有独立于HIF-1的作用机制. RT-PCR检测表明, 极度低氧1、3、5 h后IAP-2mRNA表达明显高于常氧组(t = 6.900, P<0.05), 各时间组无显著差异, 复氧后恢复基线水平. 该结果与上述IAP-2蛋白表达上调一致,初步表明IAP-2上调发生在转录水平.
结论: 极度低氧下IAP-2在人胰腺癌细胞株PC-3表达上调, 并具有独立于HIF-1的抗凋亡机制.
引文著录: 赵秋, 谷华, 杜静, 覃华, 刘南植. 低氧对胰腺癌细胞株PC-3中IAP-2表达的影响机制. 世界华人消化杂志 2005; 13(17): 2098-2102
Revised: July 1, 2005
Accepted: July 8, 2005
Published online: September 15, 2005
AIM: To investigate the expression of apoptosis inhibitory protein 2 (IAP-2) in pancreatic cancer cell PC-3 under severe hypoxia, and to explore its relation with hypoxia inducible factor 1(HIF-1).
METHODS: PC-3 cells were cultured under different conditions as follows: normoxia; 20 mL/L O2, 50 mL/L CO2, and 930 mL/L N2 for 4 h (hypoxia); 950 mL/L N2 and 50 mL/L CO2 for 1, 3, 5 h, respectively (severe hypoxia); reoxygenation after 1 h of severe hypoxia; normoxia with colalt chloride (300 µmol/L). Immunocytochemistry was used to qualitatively evaluate the expression of IAP-2 protein. After extraction of cytoplasmic and nuclear proteins, Western blot was used to quantitatively determine the expression of IAP-2 protein, which was compared with the expression of HIF-1 protein. Then the expression of IAP-2 mRNA was detected by reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS: IAP-2 protein was positively expressed in the cytoplasm of PC-3 cells. There was no significant difference between the expression levels of IAP-2 protein under normoxia and hypoxia. The expression of IAP-2 protein was markedly increased (t = 3.300, P <0.05) 1 h after severe hypoxia and remained high at 3 or 5 h. There was no significant difference among different time points (P <0.05). Reoxygenation led to basal expression of IAP-2 protein and mRNA. HIF-1 expression was undetectable in normoxic PC-3 cells, but it was induced by hypoxia. Under severe hypoxia, HIF-1 was modestly expressed, but IAP-2 was abundantly expressed. After reoxygenation, the expression of HIF-1 disappeared, and IAP-2 returned to the basal level. Colalt chloride activated HIF-1 but not IAP-2. One hour after severe hypoxia, the expression of IAP-2 mRNA was evidently higher than that under normoxia (t = 6.900, P <0.05) and remained high at 3, 5 h. There was no significant different among different time points (P >0.05).
CONCLUSION: Severe hypoxia induces the up-regulation of IAP-2 in PC-3 cells through HIF-1-independent pathways.
- Citation: Zhao Q, Gu H, Du J, Qin H, Liu NZ. Effect of hypoxia on apoptosis inhibitory protein 2 expression and its mechanism in pancreatic cancer cell line PC-3. Shijie Huaren Xiaohua Zazhi 2005; 13(17): 2098-2102
- URL: https://www.wjgnet.com/1009-3079/full/v13/i17/2098.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i17.2098
在肿瘤形成和组织缺血中缺氧是普遍存在的, 缺氧的程度决定了细胞是走向凋亡或是适应缺氧存活下来,长期的缺氧形成细胞对缺氧诱导的凋亡抵抗性,这些产生抗性的瘤体具有更强的侵袭性, 同时降低了对放疗化疗的反应. 现国外正着手研究于不同缺氧环境表达的各种调节因子对细胞凋亡、抗凋亡及增殖的影响及相互关系, 目前缺氧诱导因子1(hypoxia inducible factor 1,HIF-1)研究较多, 结论显示HIF-1在缺氧时可起到诱导凋亡或抗凋亡的作用[1-4]. 在大鼠肾近曲小管(RPTC)研究中发现, 极度低氧(近乎无氧)可诱发凋亡抑制蛋白2(apoptosis inhibitory protein 2, IAP-2)表达上调并表现出抗凋亡性, 其机制可能是IAP-2协同其他因子阻止Bak转位以保持线粒体完整性, 通过抑制caspase活化发挥抗凋亡作用, 具体机制不清[5]; 在人实体瘤中, IAP-2表达的变化和对凋亡的影响及机制探讨尚未报道. 我们选用人胰腺癌细胞株PC-3作为研究对象, 于极度低氧下观察IAP-2表达的变化, 同时观察HIF-1的表达, 以探讨缺氧时IAP-2的表达与HIF-1之间有无相关性, 为进一步研究实体瘤抗凋亡性提供新的思路及临床治疗提供可靠实验依据.
人胰腺癌细胞株PC-3由本研究所提供, DMEM高糖培养基、胎牛血清购自HyClone公司, Oxyrase购自Oxyrase公司, 六孔板、免疫组化试剂盒购自Santa Cruz公司, 兔抗人IAP-2多克隆抗体购自美国Proteintech公司, 羊抗人HIF-1mAb购自Novus公司,羊抗兔IgG购自三鹰科技公司, 羊抗小鼠IgG(H+L)购自凌飞公司, IAP-2引物合成自生工生物工程公司, PCR试剂盒购自TaKaRa公司, HEPES, NP40, EDTA, PMSF等蛋白裂解试剂,核酸裂解试剂购自Sigma公司, DTT, 丙烯酰胺, TMEMD, SDS, NC膜等Western blot试剂购自Ameresco公司, ECL发光试剂购自Pierce公司. PC-3细胞株分组孵育[6,7]: 常氧组, 20mL/L O2, 50mL/L CO2, 930mL/L N2低氧组4 h; 950mL/L N2/50mL/L CO2极度低氧组1,3,5 h, 950mL/L N2/50mL/L CO2低氧3 h后复氧,另取一组加入300 μmol/L氯化钴常氧孵育. 常氧培养时, 细胞株采用含100 mL/L胎牛血清的DMEM高糖培养基, 常规培养于50mL/L CO2/950mL/L 空气、37℃孵箱中, 待其2-3 d贴壁生长至70-80%融合时, 用胰蛋白酶消化传代.低氧培养时,将细胞株提前转入20mL/L O2/50mL/L CO2/930mL/LN2, 37℃低氧培养箱.极度低氧培养时, 先用PBS清洗细胞, 然后转入含950mL/L N2/50mL/L CO2的厌氧板,于Krebs-Ringer碳酸氢盐缓冲液中培养,该缓冲液提前经950mL/L N2/50mL/L CO2处理并按1: 10体积比(Oxyrase: 缓冲液)加入Oxyrase以除去残余O2, 复氧时, 细胞再转移到50mL/L CO2/950mL/L空气孵箱.
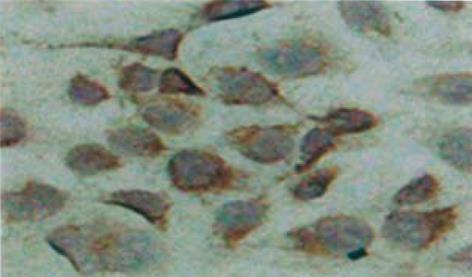
将经消毒的20 mm盖玻片置于直径90 mm培养皿中, 按2×107/L细胞接种, 3 d左右转入950mL/L N2/50mL/L CO2缺氧环境培养3 h, 以常氧培养作为对照, 行免疫细胞化学检测. PBS清洗, 冰丙酮固定, 用TritonX-100孵育, 30 mL/L H2O2孵育, 血清封闭,再加一抗IAP-2于37℃作用1 h, 二抗37℃作用0.5 h, DAB显色, 苏木素复染, 树胶封片, 光镜下观察.
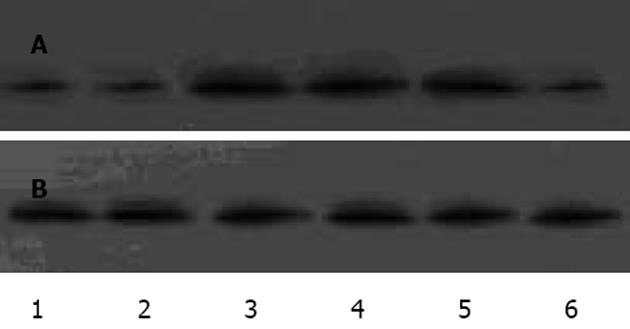
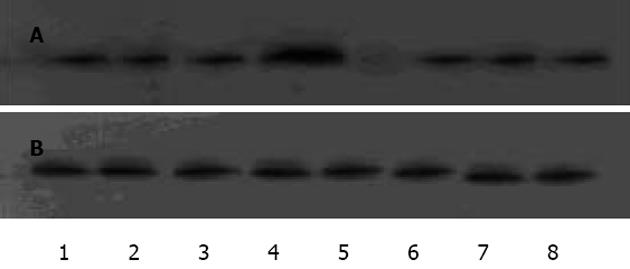
1.2.1 Westeron blot检测IAP-2,HIF-1蛋白: 收集各组细胞, 冰PBS清洗2次, 加Buffer A(含HEPES-KOH, KCl, EDTA, NP40, PMSF, Aprotinin)冰育30 min, 1300 r/min离心5 min, 上清作为胞质蛋白, 沉淀加Buffer B(含HEPES-KOH, KCl, EDTA, MgCl2, 甘油, PMSF, Aprotinin)冰育30 min, 15000 g离心30 min, 上清作为核蛋白,用考马斯亮蓝法检测蛋白浓度,其余低温保存[8]. 取各组蛋白高温变性, 100 g/L聚丙烯酰胺电泳分离约1 h, 100EV、低温2 h转至NC膜, 室温封闭1 h, 各组分别加入一抗IAP-2, HIF-1, 4℃孵育过夜, 漂洗, 加二抗, 室温孵育1 h, 漂洗, 用ECL试剂显示蛋白条带, 以β-actin作为内对照. 采用Image-Pro Plus 5.1图象分析软件测定Westeron blot条带净灰度值, 并与内参照的测定结果比较, 计算其比值, 比较各组差异以及IAP-2, HIF-1之间的关系.
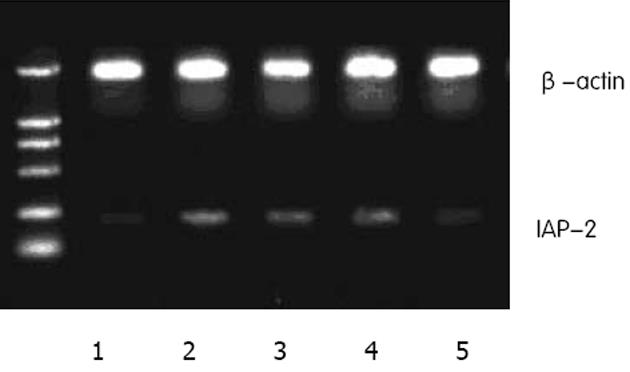
1.2.2 RT-PCR检测IAP-2 mRNA: 取常氧、极度低氧及复氧组细胞提取总RNA: PBS清洗2遍, 加入Trizol 1 mL溶液裂解. 按Trizol试剂盒说明书提取总RNA, 用分光光度仪测定RNA的浓度和纯度.cDNA的合成: 反应体系中加入提取的RNA1μL,25 mmol/LMgCl2 2 μL,10×逆转录缓冲液1 μL,10 mmol/L脱氧核苷三磷酸(dNTP)1 μL,40×107 U/L RNA酶抑制剂0.25 μL,5 ×106 U/L鸟类成髓细胞瘤病毒(AMV)逆转录酶0.5 μL,0.5 g/L寡聚脱氧核苷酸(Oligo Dt-Adaptor Primer)0.5 μL,双脱氢水3.75 μL; 42℃ 30 min,99℃ 5 min,5℃ 5 min. 低温保存. PCR检测: IAP-2基因PCR引物序列为,上游: 5′TCTTCATCGAGGACTAACCCCTAC3′,下游: 5′GCATCATCCTTTGGTTCCCAGT 3′.反应条件: 94℃变性2 min,94℃ 30 s,55℃ 30 s,72℃ 1 min,循环29次. 以β-actin为内对照检测转录效率, RT-PCR产物用15 g/L琼脂糖凝胶电泳, GoldView显色, IAP-2基因及β-actin PCR产物长度分别为200 bp, 600 bp.利用Image-Pro Plus 5.1图象分析软件测定RT-PCR条带净灰度值, 并与内参照的测定结果比较, 计算其比值.
统计学处理 数据以平均值±标准差(mean±SD)表示, 采用SPSS11.5软件进行统计学处理, 多组间均数比较用方差分析, 两组均数间比较用t检验,以P<0.05为差异具有显著性.
IAP-2蛋白在PC-3细胞中呈阳性表达, 显棕黄色, 定位于胞质, 常氧低氧表达无显著差异(图1).蛋白免疫印迹显示各组灰度值/β-actin值分别为: 常氧组(0.94±0.02)﹑20mL/L O24 h组(0.92±0.03)之间IAP-2蛋白表达均无显著性差异(t = 1.124, P>0.05),950 mL/L N2/50mL/L CO2作用1 h IAP-2表达量开始增加(1.02±0.01),与常氧组差异有显著性意义(t = 3.300,P<0.05),3 h(1.01±0.03),5 h(1.01±0.02)IAP-2持续表达, 各时间段之间无显著性差异(F = 1.194, P>0.05),复氧后(0.93±0.02)恢复低表达, 与常氧组无显著性差异(t = 2.018, P>0.05). 胞核均无表达. 可见IAP-2于极度低氧表达增高, 翻译后转至胞质通过一定机制发挥抗凋亡作用(图2).对比PC-3细胞中HIF-1表达, 常氧组HIF-1无表达, IAP-2可表达; 低氧组4 h后胞核可显示HIF-1条带, IAP-2保持基线水平; 转入极度低氧IAP-2表达明显增高, HIF-1表达(0.98±0.02)与低氧组(0.98±0.03)无显著性差异(t = 1.372, P>0.05 ); 复氧后IAP-2恢复基线表达, HIF-1不表达; 加入氯化钴4 h后HIF-1表达(0.97±0.02), 与低氧组无差异(t = 0.399, P>0.05), IAP-2保持基线, 初步表明IAP-2的表达上调有独立于HIF-1的作用机制(图3).
RT-PCR检测显示, 对比常氧组(0.79±0.01), 极度低氧1 h(0.87±0.02) IAP-2 mRNA表达明显增加, 两者具有显著性差异(t = 6.900, P<0.01), 3 h(0.86±0.01),5 h(0.88±0.01)持续表达, 各时间段无显著性差异(F = 1.068, P>0.05), 复氧(0.80±0.02)恢复表达, 与常氧组无差异(t = 0.286, P>0.05).该结果与上述IAP-2蛋白表达一致, 初步表明IAP-2上调发生于转录水平 (图4).
在实体瘤中缺氧是普遍存在的, 长期的缺氧形成细胞对缺氧诱导的凋亡抵抗性, 这些产生抗性的瘤体可能表现出更强的侵袭性, 同时降低了对放疗化疗的反应[9-11].低氧下表达的各种凋亡调节因子在诱发凋亡、对抗凋亡及促进增殖中保持着精细的平衡, 理解各种因子的作用机制及相关性对治疗实体瘤会产生新的突破[12-14]. HIF-1可发挥凋亡或抗凋亡作用[15-19].目前涉及低氧细胞凋亡或抗凋亡的相关因子及机制仍未完全阐明, 对这些机制的探讨将更有助于实体瘤的治疗[20-22].严格的缺氧(接近无氧)环境能诱发IAP-2表达上调, IAP家族(inhibitor of apoptosis family of proteins,IAPs)通过抑制caspase, 参与TNFR介导的信号转导, 与NF-κB相互作用发挥抗细胞凋亡作用[23-25].目前研究表明低氧诱导IAP-2对细胞色素C刺激的caspase活化产生抑制作用, 将IAP-2自胞质清除可恢复caspase活性[6,7].
但目前该研究仅限于大鼠, 尚未用于人类实验. 我们首次选用人胰腺癌细胞株PC-3作为研究对象,探讨实体瘤中IAP-2的表达变化及相关机制, 以加深对肿瘤细胞相关生长调节因子的认识, 为新型药物开发及临床治疗提供实验依据.本研究显示, IAP-2表达于PC-3胞质, 极度低氧环境表达较多, IAP-2在缺氧1 h后表达增加, 3-5 h未见减弱, mRNA和蛋白表达具有时间和数量的一致性, 初步显示IAP-2因子改变发生在转录水平.HIF-1蛋白常氧未见表达, 20 mL/L低氧4 h后可显示条带, 极度低氧环境条带未加深; IAP-2于常氧和20 mL/L低氧表达适量, 极度低氧明显增高; 加入氯化钴后4 hHIF-1可显示条带, IAP-2无变化, 上述结果表明HIF-1可因物理因素(低氧)或化学因素(加入氯化钴, 去铁敏等)表达, 缺氧的程度对HIF-1无明显影响, IAP-2仅在极度缺氧表达上调, 初步表明HIF-1这种介导多种基因缺氧反应的转录因子与IAP-2的表达无关, IAP-2上调及抗凋亡另有不同于HIF-1的独立作用机制. 结合对RTPC的研究成果及对人IAP-2的抗凋亡研究机制[23,26], 初步表明IAP-2在人肿瘤细胞株于极度低氧下表达增加、以独立于HIF-1的机制发挥抗凋亡作用.
通常, 在近乎无氧的条件下, 许多在适度低氧下表达的转录因子可能无法参与IAP-2的调节, 但目前研究表明, IAP-2并非在无氧下获得的唯一具有凋亡抗性的因子, IAP-2协同其他因子阻止Bax转位并保持线粒体的完整性, 可在组织缺血和肿瘤形成中维持细胞存活[27-29], 故下一步的研究应着眼于极度低氧诱导IAP-2上调的作用机理及IAP-2基因启动子元件和信号传导通路, 以及与其他因子的关联性, 并继续探寻其他低氧调节因子[26,30], 以期加深对肿瘤细胞凋亡抗性的认识, 并为进一步开展临床实验和开发新型临床抗癌药物提供可靠的理论依据和治疗靶点.
编辑: 潘伯荣 审读: 张海宁
| 1. | Piret JP, Mottet D, Raes M, Michiels C. Is HIF-1 alpha a pro- or an anti-apoptotic protein? Biochem Pharmacol. 2002;64:889-892. [DOI] |
| 2. | Semenza GL, Agani F, Feldser D, lyer N, Kotch L, Langhner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123-130. [PubMed] [DOI] |
| 3. | Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62-S67. [PubMed] [DOI] |
| 4. | Birner P, Schindl M, Obermair A, Plank c, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693-4696. [PubMed] |
| 5. | Greijer AE, Van der wall E. The role of hypoxia inducible factor 1(HIF-1)in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009-1014. [PubMed] [DOI] |
| 6. | Dong Z, Venkatachalam MA, Wang J, Patel Y, Saikumar P, Semenza GL, Force T, Nishiyama J. Up-regulation of Apoptosis Inhibitory Protein IAP-2 by Hypoxia. J Biol Chem. 2001;276:18702-18709. [DOI] |
| 7. | Dong Z, Wang JZ, Yu F, Venkatachalam MA. Apoptosis-Resistance of Hypoxic Cells: Multiple Factors Involved and a Role for IAP-2. Am J Patbol. 2003;163:663-671. [PubMed] [DOI] |
| 8. | Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura Ki, Hosokawa M, Asaka M. Constitutive Expression of Hypoxia-inducible Factor-1alpha Renders Pancreatic Cancer Cells Resistant to Apoptosis Induced by Hypoxia and Nutrient Deprivation. Cancer Reacher. 2001;61:6548-6554. |
| 9. | Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47. [DOI] |
| 10. | Lacasse EC, Baird S, Korneluk RG, Mackenzie AE. The inhibitors of apoptosis(IAPS) and their emerging role in cancer. Oncogene. 1998;17:3247-3259. [DOI] |
| 11. | Tsang RW, Fyles AW, Li Y, Raiaraman MM, Chapman W, Pintilie M, Wong CS. Tumour proliferation and apotosis in human uterine cervix carcinoma II: correlations with clinical outcome. Radiother Oncol. 1999;50:93-101. [DOI] |
| 12. | Alvarez-Tejado M, Naranio-Suarez S, Jimenez C, Carrera AC, Landazuri MO, del Peso L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276:22368-22374. [PubMed] [DOI] |
| 13. | Gottlieb RA, Granville DJ. Analyzing mitochondrial changes during apoptosis. Methods. 2002;26:341-347. [PubMed] [DOI] |
| 14. | Tsang RW, Fyles AW, Milosevic , Syed A, Pintilie M, Levin W, Manchul LA. Interrelationship of proliferation and hypoxia in carcinoma of the cervix. Int Radiat Oncol Phys. 2000;46:95-99. [PubMed] [DOI] |
| 15. | Chen J, Zhao S, Nakada K, Kuge Y, Tamaki N, Okada F, Wang J, Shindo M, Higashino F, Takeda K. Dominant-negative hypoxia-inducible factor-1 alpha reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am J Pahol. 2003;162:283-291. [PubMed] [DOI] |
| 16. | 樊 利芳, 习 路明, 陈 德基, 刘 铭球, 朱 丽琴, 李 红钢, 唐 志佼, 夏 东, 刘 绚, 陈 洪雷. 肺癌组织中缺氧诱导因 子-1α的表达及其与凋亡和增殖关系. 癌症. 2002;21:254-258. |
| 18. | Moritz W, Meier F, Stroka DM, Giuliani M, Kugelmeier P, Nett PC, Lehmann R, Candinas D, Gassmann M, Weber M. Apoptosis in hypoxic human pancreatic islets correlates with HIF-1 alpha expression. FASEBJ. 2002;16:745-747. [PubMed] [DOI] |
| 19. | Zhong H, Agani F, Baccala AA, Langhner E, Rioseco-Camacho N, Isaacs WB, Simons JW, Semenza GL. Increased expression of hypoxia inducible factor-1 alapha in rat and human prostate cancer. Cancer Res. 1998;58:5280-5284. [PubMed] |
| 20. | Zhong H, Chiles K, Feldser D, Langhner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia- inducible factor 1a expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiggenesis and therapeutics. Cancer Res. 2000;60:1541-1545. |
| 21. | Gottlieb RA, Grancville DJ. Analyzing mitochondrial changes during apoptosis. Method. 2002;26:341-347. [PubMed] [DOI] |
| 22. | Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nnt Rev Cancer. 2002;2:38-47. |
| 23. | Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rec Mol Cell Biol. 2002;3:401-441. [PubMed] [DOI] |
| 24. | LaCasse EC, Baird S, Korneluk RG, Mackenzie AE. The inhibitors of poptosis(IAPS) and their emerging role in cancer. Oncogene. 1998;17:3247-3259. [PubMed] [DOI] |
| 25. | Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239-252. [PubMed] [DOI] |
| 26. | Dong Z, Nishiyama J, Yi X, Venkatachalam MA, Denton M, Gu S, Li S, Qiang M. Gene promoter of apoptosis inhibitory protein IAP-2: identification of enhancer elements and activation by severe hypoxia. Biochem J. 2002;364:413-421. [DOI] |
| 27. | Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid,Bax, and lipids cooperate to form supramolecular openings in the outer mitochondial membrane. Cell. 2002;111:331-342. [PubMed] [DOI] |
| 28. | Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci. 2002;99:12825-12830. [PubMed] [DOI] |
| 29. | Santore MT, Mcclintock DS, Lee VY, Budinger GR, Chandel NS. Antoxia-induced apoptosis occurs through a mitochondria-dependent pathway in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L727-L734. [PubMed] [DOI] |