Copyright
©The Author(s) 2004. Published by Baishideng Publishing Group Inc. All rights reserved.
肝癌患者树突状细胞融合肝癌细胞体外诱导特异性抗肝癌免疫
王亮, 殷晓煜, 吕明德, 李宝金, 黄洁夫
王亮, 殷晓煜, 吕明德, 李宝金, 黄洁夫, 中山大学附属第一医院肝胆外科 广东省广州市 510080
ORCID number: $[AuthorORCIDs]
基金项目: 国家自然科学基金资助课题, No. 30100180.
电话: 020-87765183 传真: 020-87765183
收稿日期: 2003-11-26
修回日期: 2003-12-01
接受日期: 2003-12-16
在线出版日期: 2004-04-15
目的: 探讨肝癌(HCC)患者树突状细胞(DC)融合HCC细胞体外诱导同源T淋巴细胞产生特异性抗HCC免疫的效能.
方法: 应用人重组粒细胞/巨噬细胞集落刺激因子(rhGM-CSF)和白介素-4(rhIL-4)对肝癌患者外周血单个核细胞进行体外诱导产生树突状细胞, 流式细胞仪检测DC表面标志物表达水平, 聚乙二醇融合DC与肝癌细胞HepG2, MTT法测定融合细胞(HepG2/DC)刺激同源T淋巴细胞增生、分化能力, 细胞毒性试验检测HepG2/DC诱导的细胞毒T淋巴细胞(CTL)对HepG2的特异性杀伤作用.
结果: 体外培养1 wk后的DC高度表达CD1a, HLA-DR, CD54, CD80和CD86, 融合细胞HepG2/DC刺激同源T淋巴细胞增值能力显著高于HepG2, HepG2+DC, DC及PBS, A值分别为0.816±0.019, 0.541±0.020, 0.632±0.018, 0.564±0.018, 0.345±0.013 (P <0.05), HepG2/DC活化的CTL对HepG2具有明显的特异性杀伤作用.
结论: HCC患者外周血DC融合HCC细胞可有效诱导同源T淋巴细胞产生特异性抗HCC免疫, 可望成为一条HCC免疫治疗的有效途径.
关键词: N/A
引文著录: 王亮, 殷晓煜, 吕明德, 李宝金, 黄洁夫. 肝癌患者树突状细胞融合肝癌细胞体外诱导特异性抗肝癌免疫. 世界华人消化杂志 2004; 12(4): 774-777
Eliciting specific antitumor immunity against hepatocellular carcinoma in vitro by fusions of HCC patient-derived dendritic cells with HCC cells
Liang Wang, Xiao-Yu Yin, Ming-De Lu, Bao-Jin Li, Jie-Fu Huang
Liang Wang, Xiao-Yu Yin, Ming-De Lu, Bao-Jin Li, Jie-Fu Huang, Department of Hepatobiliary Surgery, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China
Supported by: the National Natural Science Foundation of China, No. 30100180.
Correspondence to: Dr. Xiao-Yu Yin, Department of Hepatobiliary Surgery, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China. yinxy@21cn.com
Received: November 26, 2003
Revised: December 1, 2003
Accepted: December 16, 2003
Published online: April 15, 2004
AIM: To investigate the ability of fusions of HCC patient-derived dendritic cells (DC) with HCC cells to induce autologous T lymphocytes to elicit specific immunity against HCC in vitro.
METHODS: Dendritic cells isolated from HCC patient peripheral blood were cultured and proliferated in vitro for one wk by using recombinant human granulocyte/macrophage-colony stimulating factor (rhGM-CSF) and interleukin-4 (rhIL-4). Expression of DC surface markers was assessed by flow cytometry. Fusions of DC with HepG2 cells (HepG2/DC) were achieved by polythyleneglycol (PEG). The ability of HepG2/DC to stimulate proliferation and differentiation of autologous T lymphocytes was assessed by MTT method, and the specific killing efficacy of HepG2/DC-induced cytotoxic T lymphocytes (CTL) to HepG2 was evaluated.
RESULTS: Following one wk culture, DC presented a high-level expression of CD1a, HLA-DR, CD54, CD80 and CD86. Fusions had remarkably greater ability to stimulate proliferation of autologous T lymphocytes in comparison with HepG2, HepG2+DC, DC and PBS, with an A value of 0.816±0.019 vs 0.541±0.020, 0.632±0.018, 0.564±0.018, 0.345±0.013, respectively (P<0.05). The HepG2/DC-activated CTLs showed a potent specific killing efficacy to HepG2.
CONCLUSION: Fusions of HCC patient-derived DC with HCC cells can effectively stimulate autologous T lymphocytes to elicit specific antitumor immunity against HCC, and may represent as a promising approach of immunotherapy for HCC.
Key Words: N/A
0 引言
树突状细胞(dendritic cell, DC)是人体内专职的抗原呈递细胞, 他能有效活化同源T淋巴细胞产生抗原特异性免疫应答[1-2], 利用DC呈递肿瘤抗原进而诱导特异性抗肿瘤免疫是当今肿瘤免疫治疗领域的一个研究热点[3-10], 其关键之处是如何使DC能有效呈递肿瘤抗原. DC与肿瘤细胞融合是最近发展起来的新技术[11-12], 他能确保DC在获得肿瘤细胞全部基因的基础上表达并呈递多种肿瘤抗原. 我们通过体外实验探讨DC融合肝细胞性肝癌(hepatocellular carcinoma, HCC)细胞诱导同源T淋巴细胞产生特异性细胞毒性T淋巴细胞(cytotoxic T lymphocyte, CTL)及其对HCC细胞的杀伤效能.
1 材料和方法
1.1 材料
2.5 g/L胰蛋白酶液+0.2 g/L EDTA, RPMI1640培养基购自Gibco公司; 胎牛血清购自Hyclone公司; 20 mmol/L HEPES为Sigma公司产品; 2 mmol/L谷氨酰胺购自Gibco公司; 500 mL/L聚乙二醇购自Sigma公司; 淋巴细胞分离液购自上海试剂二厂; 四甲基偶氮唑盐(MTT)为上海生工公司产品, 用0.02 mol/L PBS液配制5 g/L储存液; 重组人粒细胞/巨噬细胞集落刺激因子(rhGM-CSF), 重组人白介素-4(rhIL-4)及重组人白介素-2(rhIL-2)购自Sigma公司; 鼠抗人CD1a-FITC(异硫氰酸荧光素), HLA-DR-FITC, CD14-PE(藻红朊荧光素), CD54-PE, CD80-PE和CD86-FITC为Pharmingen及Caltag公司产品; 细胞毒性分析试剂盒(Cyto Tox 96Non-Radicative Cytotoxicity Assay Kit )为Promega公司产品; HepG2人肝癌细胞株, BEL-7402人肝癌细胞株和SMM-1990人胰腺癌细胞株均由中山大学动物实验中心提供; 流式细胞仪为美国Beckman-Coulter产品.
1.2 方法
取4例HCC患者外周血肝素抗凝, 以淋巴细胞分离液分离外周血单个核细胞(peripheral blood mononuclear cells, PBMC), 无钙镁Hanks液(pH7.2)洗涤3遍, 将细胞密度调节为109/L后加入6孔板培养2 h, 吸去悬浮细胞(淋巴细胞)另作培养, 留下贴壁细胞并加入含GM-CSF(100 g/L)、IL-4 (20 g/L)、100 mL/L胎牛血清的RPMI1640培养液, 在37 ℃、50 mL/L CO2孵箱中培养, 2-3 d半量更换培养液, 培养7 d. 将收集的淋巴细胞用含人IL-2 (20 kU/L)的RPMI1640培养液培养, 备用. 采用流式细胞仪检测DC表面CD1a, HLA-DR, CD14, CD54, CD80和CD86的表达. 肝癌细胞株HepG2经Co60照射(30Gy)后, 将DC与HepG2按3: 1的比例加入含500 mL/L聚乙二醇的RPMI1640, 混合培养5 min, 用RPMI1640缓慢稀释、洗涤, 以含rhGM-CSF(50 g/L)的RPMI1640培养液培养融合细胞(HepG2/DC) 2 d, 待用. 淋巴细胞增生试验设5组: A组: HepG2/DC与同源T淋巴细胞培养(1: 5); B组: HepG2与同源T淋巴细胞培养; C组: HepG2+DC与同源T淋巴细胞培养; D组: DC与同源T淋巴细胞培养; E组: PBS与同源T淋巴细胞培养. 各组置于96孔板混合培养48h后, 加入MTT(5 g/L)10 L继续培养8 h, 离心培养板(2 000 r/min, 10 min), 弃上清, 加DMSO 100 L/孔, 轻轻震荡, 待结晶完全溶解后再放置10 min, 于570 nm测定A值. 每组设3个复孔, 取均值. 将融合细胞和淋巴细胞按1: 10比例混合, 以含人IL-2 (20 kU/L)的RPMI1640培养液培养1 wk, 收集淋巴细胞作为CTL. 采用乳酸脱氢酶释放法测定细胞毒性, 96孔培养板每孔加入0.05 mL(细胞密度2×105/mL)HepG2、BEL-7402和SMM-1990肿瘤细胞, 按效应细胞与靶细胞10: 1, 20: 1, 40: 1的比例加入CTL(体积为0.05 mL), 每种条件设3个复孔, 并分别设立效应细胞和肿瘤细胞对照, 置于37 ℃, 50 ml/L CO2孵育6 h, 然后收集上清0.05 mL, 加入底物0.05 mL , 室温避光作用30 min, 加入0.05 mL终止液, 用酶标仪于490 nm处测定A值. 细胞毒性(%)=(效应细胞和靶细胞组A-效应细胞对照组A-自发释放组A)/(最大释放组A-自发释放组A) ×100%.
统计学处理 结果以mean±SD表示, 采用SPSS10.0作统计分析, 采用t检验.
2 结果
2.1 树突状细胞的获取及鉴定
HCC患者PBMC中的贴壁细胞培养2 d后, 可见均匀散布的细胞聚体形成并有少量悬浮细胞, 悬浮细胞体积仍较小; 继续培养3-4 d, 悬浮细胞明显增多并聚集成大小不一的细胞团; 至6-7 d, 细胞分散悬浮, 体积变大, 毛刺多而密, 呈现典型的成熟DC形态. 流式细胞仪检测结果显示, 73.7%以上的细胞表达DC标志分子CD1a, 而单核细胞标志分子CD14小于8.4%, 表明经rhGM-CSF与rhIL-4细胞因子诱导后大部分单核细胞转变成DC. 此外, DC尚高水平表达HLA-DR(86.5%), CD54(94.9%), CD80(83.1%)和CD86(75.3%)多种共刺激因子.
2.2 淋巴细胞增生试验
各组混合淋巴细胞培养结果可见融合细胞(HepG2/DC, A: 0.816±0.019a)刺激同源淋巴细胞增生能力显著高于其他各组(HepG2, A: 0.541±0.020; HepG2+DC, A : 0.632±0.018; DC, A : 0.564±0.018; PBS, A : 0.345±0.013; aP<0.05).
2.3 CTL特异性杀伤效能
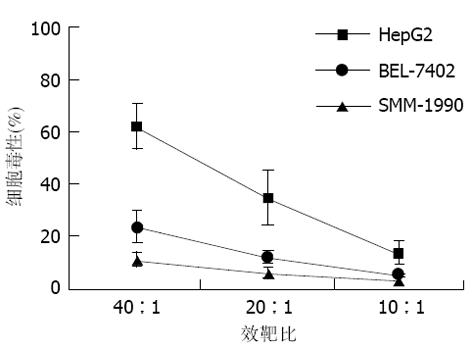
在各种不同的效靶细胞比例条件下, HepG2/DC诱导的CTL对HepG2、BEL-7402和SMM-1990的杀伤效能可见, 其对HepG2的杀伤率显著高于BEL-7402和SMM-1990, 并随效靶细胞比例增高而升高, 以40: 1时杀伤效能为最佳(图1).
3 讨论
DC是人体内最重要的专职抗原呈递细胞, 成熟的DC细胞表面表达有丰富的MHC-I, II类分子及CD54, CD80, CD86等多种共刺激分子, 能直接活化原始CD4+及CD8+T淋巴细胞, 产生抗原特异性免疫应答反应[1]. 近年来, 随着应用GM-CSF和IL-4体外成功培养、扩增DC技术的出现, 有关DC在诱导抗肿瘤免疫方面的作用受到了人们的极大关注. 已有研究[6-8,13-17]表明, 应用DC呈递肿瘤抗原可以成功诱导特异性抗黑色素瘤、甲状旁腺癌、肾癌、前列腺癌、胰腺癌、淋巴瘤及白血病等免疫应答. 利用DC诱导特异性抗肿瘤免疫的关键之处是要确保DC能有效呈递肿瘤抗原.
目前通常采用以下3种途径使DC负载并呈递肿瘤抗原: (1)与肿瘤肽裂解产物混合培养[18]. 该方法虽然简便, 但由于肿瘤抗原需经DC吞噬、加工才得以呈递, 而DC一旦成熟即散失吞噬功能, 故难以保证DC能有效地呈递肿瘤抗原; 此外, 经此途径获取的肿瘤抗原属于外源性的, DC呈递时主要活化CD4+T淋巴细胞而非诱导CTL的生成, 而后者才是抗肿瘤的主导力量[1]. (2)与肿瘤提取的mRNA混合培养[17]. 由于体外mRNA极不稳定, 在提取及被DC吞噬过程中容易遭受破坏, 不能确保在DC内能有效合成肿瘤抗原. (3)直接转染编码特异性肿瘤抗原的基因[19]. 该方法仅适用于已有确定特异性抗原的肿瘤, 且这种针对单一抗原的免疫应答不利于杀灭所有的肿瘤细胞, 因为肿瘤细胞可通过下调该基因的表达而发生"免疫逃逸". 上述三种途径使DC负载肿瘤抗原的效力往往较差, 尤其是对象HCC这样免疫原性较弱且缺乏确定特异性抗原的肿瘤, 这可能是至今为止极少见有应用上述三种途径诱导特异性抗HCC免疫研究报道的原因.
最近, Gong et al[20-21]采用细胞融合技术将人DC与人卵巢癌、乳腺癌肿瘤细胞融合, 融合的DC能有效地诱导同源T淋巴细胞产生特异性抗肿瘤CTL. 该方法的优点是融合的DC含有肿瘤细胞的全部DNA/RNA, 从而能确保DC有效地呈递各种已知及未知的肿瘤抗原, 由此可激发针对多种肿瘤抗原的特异性细胞免疫应答, 有利于杀灭更多的肿瘤细胞; 此外, 经此途径获得的肿瘤抗原为内源性抗原, DC呈递时主要诱导CD8+T淋巴细胞产生CTL免疫应答[1]. 该方法为以DC为基础的肿瘤免疫治疗提供了一条新途径. 已有研究显示[22-23], 将正常的鼠源DC与鼠肝癌细胞融合可以成功诱导同源鼠T淋巴细胞产生特异性抗肝癌免疫. HCC患者外周血中DC低表达共刺激分子, 存在一定的功能缺陷[24]. 有关此类DC诱导同源T淋巴细胞产生特异性抗HCC免疫的效能目前尚不清楚. 我们发现, HCC患者外周血DC经rhGM-CSF, rhIL-4体外共培养1 wk后不仅在形态学上趋于成熟, 而且流式细胞仪检测结果表明DC表达高水平的HLA-DR, CD54, CD80, CD86各种共刺激分子, 提示采用rhGM-CSF, rhIL-4体外培养可以改善HCC患者外周血DC的功能状态. 淋巴细胞混合培养试验证明了HepG2/DC融合细胞能有效刺激同源T淋巴细胞增生, 显著高于单纯DC和/或HepG2的刺激效能. 体外杀伤试验显示经HepG2/DC诱导产生的CTL对HepG2具有显著的特异性杀伤作用, 杀伤能力随效靶细胞比增高而增强. HCC是我国常见的肿瘤之一[25-27], 恶性程度高, 疗效差[28-33], 我们的结果表明, HCC患者外周血来源的DC与肝癌细胞融合可以有效诱导特异性抗HCC免疫应答, 从而为HCC的免疫治疗提供了一条新的可供选择途径.