修回日期: 2003-08-20
接受日期: 2003-09-18
在线出版日期: 2004-02-15
目的: 研究肝硬化再生结节和退变结节的MRI特点并以此鉴别二者及肝细胞癌(HCC).
方法: 前瞻性地研究了26例肝硬化再生结节和退变结节的MRI表现. 对26例临床疑为肝硬化的患者做了平扫MRI, 其中18例还做了Gd-DTPA增强MRI, 10例同时做了超顺磁性氧化铁(菲立磁, Feridex)增强MRI. 所有患者经穿刺或手术病理及临床影像追踪观察证实. 并将MRI表现与病理对照分析.
结果: 26例中再生结节(RN)12例, 病灶直径在0.3-1 cm; 退变结节(DN)14例, 其中8例病灶在1-3 cm, 6例病灶大于3 cm. MRI表现: 12例RN均在T1WI呈等稍高信号和T2WI等低信号, Gd-DTPA和菲立磁增强与正常肝实质呈同步强化. 14例DN的MRI表现: 5例1-3 cm结节在T1WI呈高信号和T2WI低信号, Gd-DTPA和菲立磁增强与正常肝实质呈同步强化; 另3例1-3 cm结节病灶在T1WI呈等信号, T2WI呈高信号, 在Gd-DTPA增强MRI上, 早期呈明显强化, 延迟扫描可见环行强化带, 在菲立磁增强呈高信号提示恶变, 病理上可见肝癌细胞. 6例大于3 cm的结节中2例在T1WI、T2WI均呈等高信号, 菲立磁增强MRI呈高信号, Gd-DTPA增强MRI示巨大结节较周邻正常肝组织信号高, 其中1例还可见"结中结"征, 病理上呈分化较好的HCC; 另4例大于3 cm的结节在T1WI呈高信号, T2WI呈低信号, 菲立磁强化呈低信号, Gd-DTPA增强巨大结节无强化, 较周邻正常肝组织信号低, 有时可见血管经过巨大结节表面. 此外, Gd-DTPA增强的时间信号强度曲线显示: RN和良性DN与周邻正常肝组织表型近似, 呈缓进缓出; DN恶变时, 呈快进快出表型, 与正常肝组织不同.
结论: 肝硬化再生结节(RN)在MRI上能较好地与HCC鉴别, 但较难区别于良性退变结节(DN). 良性退变结节在T2WI不呈高信号, 以此区别HCC. 此外, 退变结节在Gd-DTPA增强上出现环行包膜强化, 时间信号强度曲线呈快进快出表型, 菲立磁增强上T2WI呈高信号, 提示有恶变.
引文著录: 徐海波, 孔祥泉, 熊茵, 冯敢生. MRI评估肝硬化再生结节和退变结节. 世界华人消化杂志 2004; 12(2): 385-389
Revised: August 20, 2003
Accepted: September 18, 2003
Published online: February 15, 2004
AIM: To study MR features of the regenerative nodule (RN) and dysplastic nodule (DN) in the cirrhotic liver.
METHODS: MRI was performed in 26 cases of suspected cirrhotic liver with RN and DN. Additional enhanced MRI with administration of Gd-DTPA on T1WI was performed in 18 of 26 cases. Meanwhile in 10 of 18 both Gd-DTPA and SPIO (Feridex) enhancement were underwent one day apart. All patients were confirmed by aspiration biopsy or histopathology. MRI was compared to the pathological findings.
RESULTS: In 26 cases, there were 12 cases of regenerative nodules measuring 0.3-1cm in size, and 14 dysplastic nodules including 8 nodules measuring ≥1 cm and <3 cm in size, and 6 nodules measuring ≥3 cm. Their MR appearances were as followings: nodules with <1 cm in size showed isointensity on T1WI and hypointensity on T2WI, of which the intensity was as isointense as the surrounding hepatic parenchyma on enhanced MRI with administration of Gd-DTPA or SPIO. In 8 cases with nodules measuring 1-3 cm in size, 5 cases appeared hyperintense on T1WI and hypointense on T2WI as well as the enhancement as that of nodules with <1 cm in size; the other 3 cases appeared hypointense on T1WI and hyperintense on T2WI, and were enhanced after administration of Gd-DTPA but hyperintense on SPIO enhancing MRI, which indicated malignant transformation of dysplastic nodule into hepatocellular carcinoma (HCC) arising from hepatic nodule on histopathology. In 6 cases of nodules measuring >3 cm in size, 2 cases appeared hyperintense compared to the surrounding hepatic parenchyma on T1, T2WI and enhanced MRI, one of which was documented "nodule within a nodule" on T2WI. The 2 cases were demonstrated well-differentiated HCC. The other 4 cases showed hyperintense on T1WI, and hypointense on T2WI and enhanced MRI. Sometimes, normal vessels were seen to pass through the surface of macroregenerative nodule. Additionally, RN and DN had the same pattern of the time-signal intensity course as the normal surrounding hepatic parenchyma, but malignant transformation of DN appeared fast wash-in and wash-out.
CONCLUSION: RN of cirrhosis has features on MRI that usually allow distinction from HCC but not always from DN. A helpful distinction between HCC and DN is that the latter is almost never hyperintense on T2WI. Additionally, the followings indicate malignant transformation of DN when DN appears a ring like enhancement after injection of Gd-DTPA, and fast wash-in and wash-out as well as hyperintensity on SPIO enhanced MRI.
- Citation: Xu HB, Kong XQ, Xiong Y, Feng GS. MRI features of regenerative and dysplastic nodules in cirrhotic liver. Shijie Huaren Xiaohua Zazhi 2004; 12(2): 385-389
- URL: https://www.wjgnet.com/1009-3079/full/v12/i2/385.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i2.385
肝硬化结节可由良性再生结节(regenerative nodule, RN)到癌前病变的退变结节(dysplastic nodule, DN)以及进一步演变成肝细胞癌[1-2]. 因而对上述结节的早期发现, 早期诊断以及追踪评估无疑对治疗方案的制订和预后的改善、评估具有重要意义. 有关肝硬化结节的不同影像表现已有国内外报道[3-12], 但认为超声对肝硬化内的小结节灶定性不可靠, 同时认为尽管CT和MR有利于肝硬化患者肝内局灶结节的检测和定性诊断, 但所用的CT和MR成像技术对这些肝内结节灶仍不敏感或鉴别癌前病变与肝细胞癌仍有疑问[13-16], 且未对RN和癌前病变的DN在MRI上的表现进行描述和鉴别. 目前, MR无疑是对慢性肝病检测最有效的影像方法, 信号强度和形态变化有利于MRI对慢性肝病的诊断和不同病因的鉴别及病变程度分级或分期, 并且现有的快速MRI扫描序列能提供动态增强评估有利于提高局灶病变的定性诊断, 包括对RN、癌前病变的DN和小的肝细胞癌的鉴别成为可能[17-18]. 我们通过不同的磁共振成像序列结合使用不同的磁共振对比剂寻找二者MRI的特点以此鉴别二者及HCC.
前瞻性地研究了26例肝硬化再生结节和退变结节的MRI表现. 对26例临床疑为肝硬化的患者做了平扫MRI, 其中18例还做了Gd-DTPA增强MRI, 10例同时做了超顺磁性氧化铁(菲立磁, Feridex)增强MRI. 所有患者经穿刺或手术病理及临床影像追踪观察证实.
所有患者均用德国西门子超导1.5T MR仪(mag-netom-vision, siemens, erlangen, germany)和体部相控阵(phase-array)线圈进行肝脏扫描. T1WI采用梯度回波同反相位序列, T2WI采用HASTE和T2* EPI及T2* FLASH 小角度小于45o序列. 层厚6-8 mm, 间距1-2 mm. 进行超顺磁性氧化铁(菲立磁, Feridex)增强MRI的方法为: 剂量0.56 mg Fe/kg, 相当于0.05 mL/kg菲立磁, 50 g/L葡萄糖稀释后经过特制5 μm孔径的过滤器静脉滴注, 2-4 mL/min, 全程不少于30 min, 注射菲立磁后30 min, 1 h, 3 h, 6 h重复T2WI及T2*序列扫描. Gd-DTPA动态增强MRI的方法为: 剂量0.2 mmol/kg, 经肘前静脉以3 mL/s速度团注连续MRI扫描延至5 min; 然后取同层面肝硬化结节、正常肝组织、腹主动脉和脾组织兴趣区(ROI)作Gd-DTPA动态增强的时间信号强度曲线. 图像分析由2名有经验高年资医师执行并与病理结果对照分析.
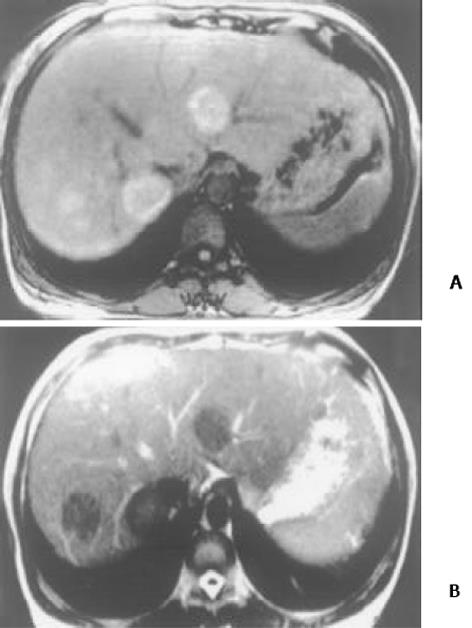
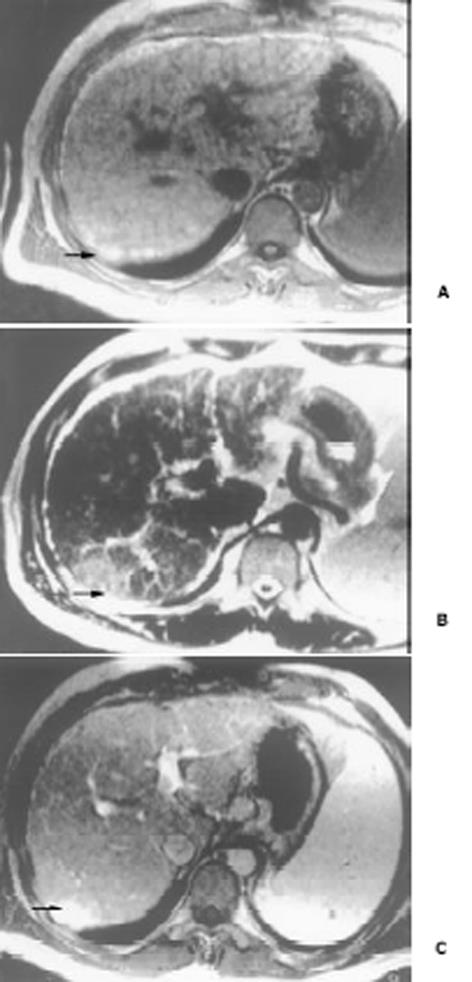
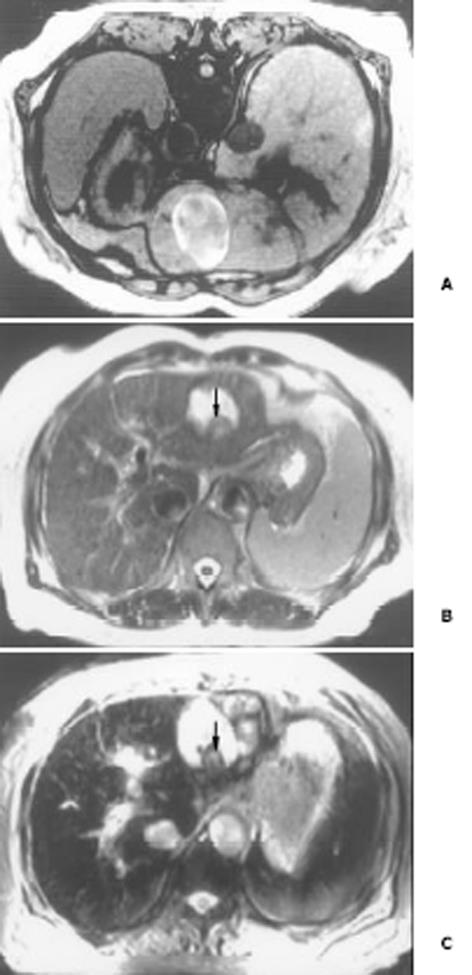
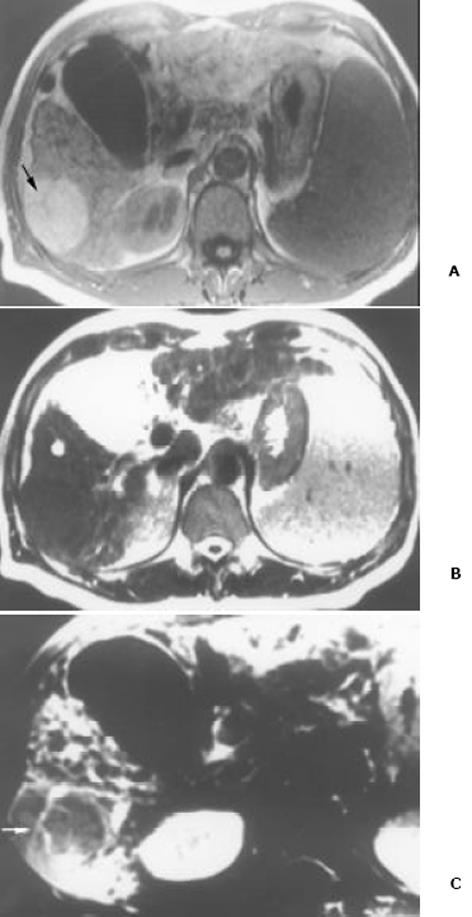
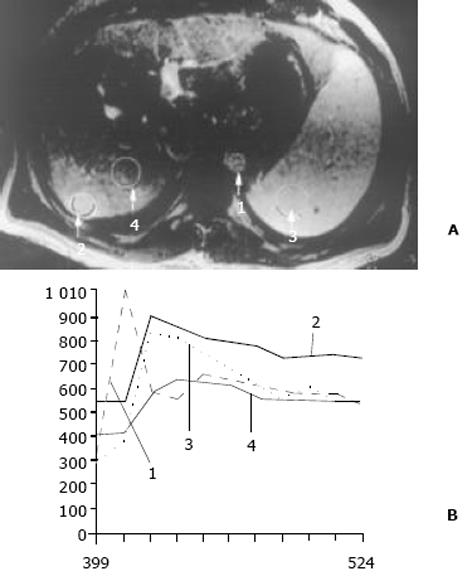
26例中再生结节(RN)12例, 病灶直径在0.3-1 cm, 病理上呈局灶性增生的肝实质小岛; 退变结节(DN)14例, 其中8例病灶在1-3 cm, 6例病灶大于3 cm. 病理上, 低度(low grade)DN含有肝细胞, 轻度异常, 无结节或细胞异型性, 但含有大量细胞发育不良; 高度(high grade)DN含有局灶或广泛结构异常或细胞异型性. MRI表现: 12例RN病灶均在T1WI呈等稍高信号和T2WI等低信号, Gd-DTPA和菲立磁增强与正常肝实质呈同步强化. 14例DN的MRI表现: 5例1-3 cm结节在T1WI呈高信号和T2WI低信号(图1), Gd-DTPA和菲立磁增强与正常肝实质呈同步强化; 另3例1-3 cm结节病灶在T1WI呈等信号, 在T2WI呈高信号, 在Gd-DTPA增强MRI上, 早期呈明显强化, 延迟扫描可见环行强化带(图2), 其时间信号强度曲线亦与正常肝组织曲线表现不同, 此外在菲立磁增强T2WI中呈高信号提示恶变, 病理显示为产生于肝结节灶上的肝细胞癌. 6例大于3cm的结节中2例在T1WI、T2WI均呈等高信号, 菲立磁增强MRI呈高信号, Gd-DTPA增强MRI示巨大结节较周邻正常肝组织信号高, 其中1例还可见"结中结"征(图3), 病理上呈分化较好的HCC; 另4例大于3 cm的结节在T1WI呈高信号, 在T2WI呈低信号, 菲立磁强化呈低信号, Gd-DTPA增强巨大结节无强化, 较周邻正常肝组织信号低, 有时可见血管经过巨大结节表面(图4).
HCC影像诊断较复杂, 其主要原因是影像表现多样化并常与再生结节(RN)和退变结节(DN)共存. 目前对HCC有效治疗方法有手术切除、肝移植、局部抑制切除治疗(focal ablation therapy), 但这取决于HCC的早期诊断[14]. 对影像学而言关键任务是首先能早期发现可疑病变, 然后是能鉴别良性结节、良恶性临界结节和恶性结节. 对有高风险癌变的肝硬化患者采用超声和甲胎蛋白(AFP)检查筛选是有益的, 但对小HCC与良性或退变结节的鉴别却不可靠[17].
本研究显示再生结节(RN)在T1WI呈等高信号, 在T2WI呈等低信号, 与Koslow et al[19]报道不完全一致, 即RN常在T1WI和T2WI呈等高信号, 较少在T1WI呈稍高信号和T2WI呈低信号. 这可能与本研究显示的小于0.3 cm RN病例少有关. RN 之所以在T2WI上呈低信号, 可能与含铁血黄色素沉着或其周围的纤维间隔有关[20]. 含铁血黄色素能有效缩短T2, 降低T2信号, 纤维间隔则由于炎性反应或扩张的血管使含水量增加而形成小环形或网状高信号影, 从而使RN呈相对低信号[21]. 退变结节(DN)可分为低度(low-grade)和高度(high-grade)两型, 后者被认为是一种癌前或临界病变[22-24], 在15-25%的肝硬化患者中发现此结节[22]. DN在显微镜下呈嗜碱胞质增多, 核和核仁增大, 有时可见微腺泡形成和纤维间隔内肝细胞增生, 但这些变化不足以诊断HCC[25]. 当DN中含有HCC结节灶时, 其倍增时间小于3 mo, 并且癌灶仅在显微镜下可见时, 无论在活体或离体组织标本上MRI常难以检测到[26]. DN在MRI T1WI呈高或等信号, 在T2WI呈等或低信号, 这两种信号结合考虑可作为DN的特征性表现. 但此信号特征与小HCC(小于2 cm) 有重叠或相似[27]. 二者常表现出T1WI上呈高信号, T2WI呈低信号. 而肝细胞癌在T2WI呈稍高信号为其特征性表现, 故HCC与DN区别在于: DN几乎在T2WI不呈高信号, 也不含有真正包膜. DN未恶变时, 本研究显示与RN在平扫和Gd-DTPA或菲立磁增强MRI上信号强度变化一致, 亦与周邻正常肝组织一致, 并且铁质沉着的RN (siderotic regenerative nodule)与铁质沉着的DN(siderotic dysplastic nodule)亦难以鉴别[28]. 但通过Gd-DTPA或菲立磁增强可帮助区别DN与小HCC, 并且Gd-DTPA增强MRI在显示恶性肝肿瘤病变优于菲立磁增强, 尤其是肝硬化患者[29-34]. 本研究中有2例大于3 cm的结节在T1WI、T2WI均呈等高信号, 常被认为胶样囊肿或退变结节囊变, 经菲立磁增强MRI呈高信号, Gd-DTPA增强MRI示巨大结节较周邻正常肝组织信号高, 病检显示为分化较好的HCC. 同样另3例1-3 cm DN提示有恶变, 最后得到病理证实, 也显示在Gd-DTPA增强MRI上, DN呈明显强化, 并有环行强化带提示有包膜形成, 其时间信号强度曲线亦呈快进快出表现, 提示DN的动脉血供发生变化, 与Krinsky et al[35]报道的病理结果一致, 即低度铁质沉着的DN不成对的动脉明显较铁质沉着的RN多. 另一方面, 当癌灶增大时, 出现典型的MRI"结中结"征象, 即在T2WI上显示低信号结节中出现高信号灶. 此时即使血液检查(甲胎蛋白)或细胞学穿刺检查呈阴性, 也应采取及时治疗或追踪观察. Kaji et al报道即使对高度DN切除后, 对这些患者随访观察发现其HCC发生的危险性仍较高. 这提示对高度DN积极治疗的患者还应注意随访观察. 此外, 最近Shimizu et al对208例肝硬化或慢性肝炎患者小于2 cm的肝结节病灶进行系列动态增强MRI检查, 发现首次检查时结节灶在动脉期有强化, 但间隔至少12 mo后动态增强MRI复查显示这些病灶无间隔生长, 有的反而消失, 并认为尽管这些病灶呈圆形或卵圆形, 但常为假病灶(pseudolesion)而不是HCC. 由此说明, 对肝硬化内结节病变, 尤其是RN和DN一定要随访动态观察.
编辑: N/A
| 1. | Krinsky GA, Lee VS, Theise ND. Focal lesions in the cirrhotic liver: high resolution ex vivo MRI with pathologic correlation. J Comput Assist Tomogr. 2000;24:189-196. [PubMed] [DOI] |
| 2. | Efremidis SC, Hytiroglou P. The multistep process of hepatocarcinogenesis in cirrhosis with imaging correlation. Eur Radiol. 2002;12:753-764. [PubMed] [DOI] |
| 3. | Mortele KJ, Ros PR. MR imaging in chronic hepatitis and cirrhosis. Semin Ultrasound CT MR. 2002;23:79-100. [PubMed] [DOI] |
| 4. | de Lédinghen V, Laharie D, Lecesne R, Le Bail B, Winnock M, Bernard PH, Saric J, Couzigou P, Balabaud C, Bioulac-Sage P. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? A prospective study of 88 nodules in 34 patients. Eur J Gastroenterol Hepatol. 2002;14:159-165. [PubMed] [DOI] |
| 5. | Mikami S, Kubo S, Hirohashi K, Shuto T, Kinoshita H, Nakamura K, Yamada R. Computed tomography during arteriography and arterial portography in small hepatocellular carcinoma and dysplastic nodule: a prospective study. Jpn J Cancer Res. 2000;91:859-863. [PubMed] [DOI] |
| 7. | Fracanzani AL, Burdick L, Borzio M, Roncalli M, Bonelli N, Borzio F, Maraschi A, Fiorelli G, Fargion S. Contrast-enhanced Doppler ultrasonography in the diagnosis of hepatocellular carcinoma and premalignant lesions in patients with cirrhosis. Hepatology. 2001;34:1109-1112. [PubMed] [DOI] |
| 8. | Coakley FV, Schwartz LH. Imaging of hepatocellular carcinoma: a practical approach. Semin Oncol. 2001;28:460-473. [PubMed] [DOI] |
| 9. | Sahani DV, O'Malley ME, Bhat S, Hahn PF, Saini S. Contrast-enhanced MRI of the liver with mangafodipir trisodium: imaging technique and results. J Comput Assist Tomogr. 2002;26:216-222. [PubMed] [DOI] |
| 10. | Murakami T, Mochizuki K, Nakamura H. Imaging evaluation of the cirrhotic liver. Semin Liver Dis. 2001;21:213-224. [PubMed] [DOI] |
| 11. | Bartolozzi C, Donati F, Cioni D, Crocetti L, Lencioni R. MnDPDP-enhanced MRI vs dual-phase spiral CT in the detection of hepatocellular carcinoma in cirrhosis. Eur Radiol. 2000;10:1697-1702. [PubMed] [DOI] |
| 12. | Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med. 2001;20:99-104. [PubMed] [DOI] |
| 13. | Kanematsu M, Hoshi H, Yamada T, Murakami T, Kim T, Kato M, Yokoyama R, Nakamura H. Small hepatic nodules in cirrhosis: ultrasonographic, CT, and MR imaging findings. Abdom Imaging. 1999;24:47-55. [PubMed] [DOI] |
| 14. | Martín J, Puig J, Darnell A, Donoso L. Magnetic resonance of focal liver lesions in hepatic cirrhosis and chronic hepatitis. Semin Ultrasound CT MR. 2002;23:62-78. [PubMed] [DOI] |
| 15. | Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327-336. [PubMed] [DOI] |
| 16. | Freeny PC, Grossholz M, Kaakaji K, Schmiedl UP. Significance of hyperattenuating and contrast-enhancing hepatic nodules detected in the cirrhotic liver during arterial phase helical CT in pre-liver transplant patients: radiologic-histopathologic correlation of explanted livers. Abdom Imaging. 2003;28:333-346. [PubMed] [DOI] |
| 17. | Ward J, Robinson PJ. How to detect hepatocellular carcinoma in cirrhosis. Eur Radiol. 2002;12:2258-2272. [PubMed] [DOI] |
| 18. | Hussain SM, Zondervan PE, IJzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22:1023-1036; discussion 1037-1039. [PubMed] [DOI] |
| 19. | Koslow SA, Davis PL, DeMarino GB, Peel RL, Baron RL, Van Thiel DH. Hyperintense cirrhotic nodules on MRI. Gastrointest Radiol. 1991;16:339-341. [PubMed] [DOI] |
| 20. | Murakami T, Kuroda C, Marukawa T, Harada K, Wakasa K, Sakurai M, Monden M, Kasahara A, Kawata S, Kozuka T. Regenerating nodules in hepatic cirrhosis: MR findings with pathologic correlation. AJR Am J Roentgenol. 1990;155:1227-1231. [PubMed] [DOI] |
| 21. | Murakami T, Kim T, Nakamura H. Hepatitis, cirrhosis, and hepatoma. J Magn Reson Imaging. 1998;8:346-358. [PubMed] [DOI] |
| 22. | Hytiroglou P, Theise ND. Differential diagnosis of hepatocellular nodular lesions. Semin Diagn Pathol. 1998;15:285-299. [PubMed] |
| 23. | Borzio M, Fargion S, Borzio F, Fracanzani AL, Croce AM, Stroffolini T, Oldani S, Cotichini R, Roncalli M. Impact of large regenerative, low grade and high grade dysplastic nodules in hepatocellular carcinoma development. J Hepatol. 2003;39:208-214. [PubMed] [DOI] |
| 24. | An HJ, Illei P, Diflo T, John D, Morgan G, Teperman L, Theise N. Scirrhous changes in dysplastic nodules do not indicate high-grade status. J Gastroenterol Hepatol. 2003;18:660-665. [PubMed] [DOI] |
| 25. | Wada K, Kondo F, Kondo Y. Large regenerative nodules and dysplastic nodules in cirrhotic livers: a histopathologic study. Hepatology. 1988;8:1684-1688. [PubMed] [DOI] |
| 26. | Ito K, Mitchell DG, Gabata T, Hann HW, Kim PN, Fujita T, Awaya H, Honjo K, Matsunaga N. Hepatocellular carcinoma: association with increased iron deposition in the cirrhotic liver at MR imaging. Radiology. 1999;212:235-240. [PubMed] [DOI] |
| 27. | Sadek AG, Mitchell DG, Siegelman ES, Outwater EK, Matteuccí T, Hann HW. Early hepatocellular carcinoma that develops within macroregenerative nodules: growth rate depicted at serial MR imaging. Radiology. 1995;195:753-756. [PubMed] [DOI] |
| 28. | Krinsky GA, Lee VS, Nguyen MT, Rofsky NM, Theise ND, Morgan GR, Teperman LW, Weinreb JC. Siderotic nodules at MR imaging: regenerative or dysplastic? J Comput Assist Tomogr. 2000;24:773-776. [PubMed] [DOI] |
| 29. | Matsuo M, Kanematsu M, Itoh K, Ito K, Maetani Y, Kondo H, Kako N, Matsunaga N, Hoshi H, Shiraishi J. Detection of malignant hepatic tumors: comparison of gadolinium-and ferumoxide-enhanced MR imaging. AJR Am J Roentgenol. 2001;177:637-643. [PubMed] [DOI] |
| 30. | Sugihara S, Suto Y, Kamba M, Ogawa T. Comparison of various techniques of iron oxide-enhanced breath-hold MR imaging of hepatocellular carcinoma. Clin Imaging. 2001;25:104-109. [PubMed] [DOI] |
| 31. | Fujita T, Ito K, Honjo K, Okazaki H, Matsumoto T, Matsunaga N. Hepatic parenchymal enhancement in the cirrhotic liver: evaluation by triple-phase dynamic MRI. Abdom Imaging. 2002;27:29-33. [PubMed] [DOI] |
| 32. | Ward J, Guthrie JA, Scott DJ, Atchley J, Wilson D, Davies MH, Wyatt JI, Robinson PJ. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology. 2000;216:154-162. [PubMed] [DOI] |
| 33. | Takeshita K, Nagashima I, Frui S, Takada K, Yamauchi T, Harasawa A, Oba H, Kohtake H, Tanaka H, Suzuki S. Effect of superparamagnetic iron oxide-enhanced MRI of the liver with hepatocellular carcinoma and hyperplastic nodule. J Comput Assist Tomogr. 2002;26:451-455. [PubMed] [DOI] |
| 34. | Zheng WW, Zhou KR, Chen ZW, Shen JZ, Chen CZ, Zhang SJ. Characterization of focal hepatic lesions with SPIO-enhanced MRI. World J Gastroenterol. 2002;8:82-86. [PubMed] [DOI] |
| 35. | Krinsky GA, Zivin SB, Thorner KM, Lee VS, Theise ND, Weinreb JC. Low-grade siderotic dysplastic nodules: determination of premalignant lesions on the basis of vasculature phenotype. Acad Radiol. 2002;9:336-341. [PubMed] [DOI] |