修回日期: 2003-01-25
接受日期: 2003-02-19
在线出版日期: 2003-09-15
研究联合应用胃泌素拮抗剂和胞嘧啶脱氨酶(CD)的基因治疗对结肠癌细胞的杀伤作用.
脂质体法转染G1CEACDNa至LoVo细胞. 用含1 mmol/L 5-FC或/和1× 10-8 mmol/L CI-988的100 ml/L胎牛血清的RPMI1640培养基培养细胞, 测生长曲线; MTT法测细胞杀伤率; 透射电镜观察细胞的超微结构; 流式细胞仪annexin V和PI双重染色行凋亡相关检查; Balb/c裸鼠背部皮下接种CD+ LoVo细胞, 5-FC (500 mg/kg)腹腔注射或/和CI-988 (10 mg/kg)灌胃处理20 d, 处死裸鼠后肿瘤称重, 病理切片HE染色, 瘤组织透射电镜检查.
用5-FC或/和CI-988处理6d后CD+ LoVo细胞抑制率分别为90 %和50 %, 二者合用于3d和6d抑制率分别为40 %和97 %; MTT法检测联合应用5-FC和CI-988较单用任何一种处理对CD+ LoVo细胞杀伤作用更强, 有统计学差异(0.53±0.05 vs 0.49±0.02, 0.38±0.06, F =29.5536, n=5, P <0.01); 电镜和流式细胞仪分别显示CD/5-FC和CI-988结合处理72 h后细胞有凋亡现象; 用5-FC (500 mg/kg)腹腔注射和CI-988 (10 mg/kg)灌胃处理荷瘤裸鼠均可出现明显的抑瘤作用, 抑瘤率分别为69.4 %和49.5%, 二者合用抑瘤较单用任一种处理更明显, 有统计学意义(0.42±0.12 vs 0.69±0.11, 1.22±0.22, F=33.1 709, n=5, P<0.05), 抑瘤率达81.5 %, 电镜下见瘤组织内有凋亡小体.
联合应用胃泌素拮抗剂CI-988可以提高CD/5-FC对结肠癌细胞的杀伤作用. 凋亡相关过程可能是这种协同作用的原因.
引文著录: 王小军, 马庆久, 赖大年, 黎成金, 李金茂, 武永忠, 王青. 胃泌素拮抗剂增加CD自杀基因对结直肠癌细胞的杀伤作用. 世界华人消化杂志 2003; 11(9): 1385-1388
Revised: January 25, 2003
Accepted: February 19, 2003
Published online: September 15, 2003
To investigate the killing effect of cytosine deaminase/5-fluorocytosine on colorectal carcinoma when combined with gastrin receptor antagonist.
Plasmid G1CEACDNa was transferred into the LoVo cell line using liposomes method. The growth curve of cells was observed when cultured with 5-FC (1 mmol/L) or/and CI-988 (1×10-8mmol/L) in RPMI-1 640+100 ml/L fatal bovine serum. The killing efficiency was measured by MTT method. The submicroscopic structure of cells was observed by electron microscope, apoptosis was verified by a flow cytometer . CD+ LoVo cells were were. inoculated s.c. in athymic nude mice followed by 5-fluorocysine or/and CI-988 treatment for 20 d. these nude mice were killed with their tumor weight determined. Then tumor tissue was stained with hematoxylin and eosin, the submicroscopic structure of cells was observed by electron microscope.
After treated with 5-FC or CI-988,the inhibition rate of CD+ LoVo cells was 90 % and 50 %, respectively. When c these two reagents were used in combination, the inhibition rate was 40 % and 97 % on 3 d and 6 d, respectively. By MTT method, combination of CD/5-FC and CI-988 possessed superior killing effect in comparison to single therapy (0.53±0.05 vs 0.49±0.02, 0.38±0.06, F =29.5536, n =5, P <0.01). Electron microscope and flow cytometer verified that cells were apoptosized after exposed to 5-FC (1 mmol/L) and CI-988(1×10-8 mmol/L) 72 h. Significant anti-tumor effect was observed in nude mice bearing CD+ LoVo cells followed with 5-FC(500mg/kg body weight i.p. injection per day) or CI-988 (10 mg/kg per day orally), the inhibition rate were 69.4% and 49.5%,respectively. When these two reagents were used in combination, the inhibition rate was 81.5 % that was higher compared to single therapy (0.42±0.12 vs 0.69±0.11, 1.22±0.22, F =33.1709, n =5, P <0.05).There were apoptosis bodies in submicroscopic structure.
Gastrin receptor antagonist canelevate the killing effect of CD/5-FC on colorectal carcinoma. Apoptosis is possibly one of the reasons of the synergistic action.
- Citation: Wang XJ, Ma QJ, Lai DN, Li CJ, Li JM, Wu YZ, Wang Q. Gastrin receptor antagonist combined with cytosine deaminase suicide gene therapy enhances killing of colorectal carcinoma. Shijie Huaren Xiaohua Zazhi 2003; 11(9): 1385-1388
- URL: https://www.wjgnet.com/1009-3079/full/v11/i9/1385.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i9.1385
细胞内转染胞嘧啶脱氨酶(cytosine deaminase, CD)基因, 随后给予5-氟胞嘧啶(5-FC), 是近年来发展起来的肿瘤的自杀基因疗法中研究较为广泛的一种方案.大量基础和临床实验[1-5]已经证明CD/5-FC对结直肠癌治疗有效. 但是, 基因治疗普遍存在转染效率低的问题限制了其应用前景.胃泌素有刺激肠道上皮增生的能力[6-9], 胃泌素及其受体在结肠肿瘤细胞生长调节中起着重要作用, 胃泌素拮抗剂对结肠癌的生长及转移有明显抑制作用. 我们联合应用CD/5-FC和胃泌素拮抗剂对结肠癌细胞生长的影响.
CEA启动子调控CD基因表达的逆转录病毒载体G1CEACDNa由第二军医大学长海医院崔龙教授惠赠; 大肠癌LoVo细胞株由上海医科大学瑞金消化外科研究所提供; Balb/c裸鼠由第四军医大学实验动物中心提供; 脂质体lipofectamineTM 2000 (LF 2000)为Invitrogen公司产品; G 418为Gibco公司产品; 5-氟胞嘧啶(5-FC), 胃泌素拮抗剂CI-988, MTT为Sigma公司产品; RPMI1640培养基, 胎牛血清为Promega公司产品.
大肠癌细胞的转染在6孔培养板中分别接种106个LoVo细胞, 待细胞生长至80 %融合时, 用无血清RPMI1640培养基轻洗细胞并置换原来的培养液, 再加入含4 mg质粒DNA250 mL和LF2000的无血清RPMI1640培养基10 mL培养24 h, 以含400 mg/L-1 G 418及100 mL/L-1胎牛血清的RPMI1640培养基传代培养转染细胞, 选择培养14 d, 筛选抗性克隆并扩增. 将CD+ LoVo分别接种至4 块24孔板中 (108/L), 依次分为4组. 实验组1用含1 mmol/L 5-FC的100 ml/L胎牛血清的RPMI1640培养基培养; 实验组2用含1×10-8 mmol/L CI-988的100 ml/L胎牛血清的RPMI1640培养基培养; 实验组3用含1 mmol/L 5-FC和1×10-8 mmol/L CI-988的100 ml/L胎牛血清的RPMI1640培养基培养; 对照组用 100 ml/L胎牛血清的RPMI1640培养基正常培养. 每天胰酶消化法计数每组4孔, 绘制细胞在不同条件下的生长曲线, 计算生长抑制率=(1-实验组细胞计数平均值/对照组同期细胞计数平均值)×100 %. 将CD+ LoVo接种至24孔板中(108/L), 细胞分为4组(分组情况同前), 每组设5个复孔. 于培养72 h行MTT法检测490 nm处吸光度A值, 各组A值进行方差分析. 计算细胞存活率=A实验组均数/A对照组均数×100 %.
CD+ LoVo培养至对数生长期, 加入含1 mmol/L 5-FC和1×10-8 mmol/L CI-988的100 ml/L胎牛血清的RPMI1640培养基, 治疗72 h后, 收集细胞1×107, 2.5 %胰蛋白酶消化, 用无血清RPMI1640培养基洗涤2次, 2 000 r/min-1离心10 min, 贴壁加入固定液, 送电镜室包埋、切片, 透射电镜下检查. 用60 mL培养瓶2个将CD+ LoVo细胞培养至对数生长期, 实验组加入含1 mmol/L 5-FC和1×10-8 mmol/L CI-988的100 ml/L胎牛血清的RPMI1640培养基, 培养72 h后用橡皮刮收集细胞, 离心(300 g, 5 min)去上清后, 重新悬浮于1 mL的annexin V-FITC染色液, 室温下染色5 min. 在细胞悬液中加入PI保存液10 mL, 室温下染色5 min后上机检测.
取8-12周龄Balb/c裸鼠20只, 背部皮下接种CD+ LoVo细胞(5×106cell in 0.2 mL PBS), 成瘤后按体重大小编号, 随机化分为4组. 实验组1给予5-FC (500 mg/kg)腹腔注射, 实验组2给予CI-988 (10 mg/kg)灌胃, 实验组3给予5-FC (500 mg/kg)腹腔注射并CI-988 (10 mg/kg)灌胃, 对照组未给任何处理. 给药均为1次/d, 连续20 d, 处死裸鼠分离出肿瘤组织分别称重, 然后用40 g/L甲醛固定, 二期行组织切片HE染色. 其中实验组3处死裸鼠后立即取微量肿瘤组织戊二醛固定, 二期行透射电镜检测. 计算抑瘤率= (1-实验组瘤重平均值/对照组瘤重平均值)×100 %.
统计学处理 应用SPLM统计软件行方差分析统计学处理.
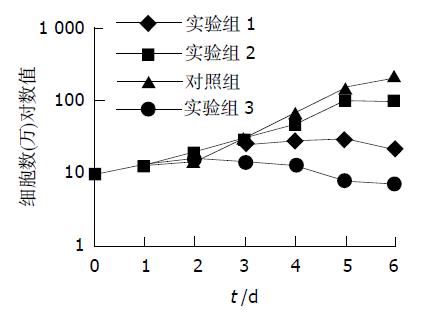
实验组1于处理后3 d细胞开始出现死亡, 6 d时生长抑制率达90 %以上; 实验组2于处理后3 d细胞出现形态变化活性降低, 4 d可见部分细胞开始死亡, 6 d时细胞生长抑制率达50 %; 实验组3于处理后2 d即表现为细胞变形、空泡、折光性差, 3 d可见明显死亡现象, 抑制率达40 %, 6 d抑制率达97 %(图1). 实验组1, 实验组3细胞杀伤作用明显, 细胞杀伤率分别为28.7 %和47.1 %, A值较对照组行方差分析有显著性差异(P <0.01); 实验组2(7.8 %)未见明显杀伤细胞效应; 与对照组A值(0.53±0.05)无显著性差异(P >0.05); 实验组3 (0.28±0.04)较实验组1 (0.38±0.06)、实验组2 (0.49±0.02)杀伤作用更明显, 方差分析A值有显著性差异(P <0.01).
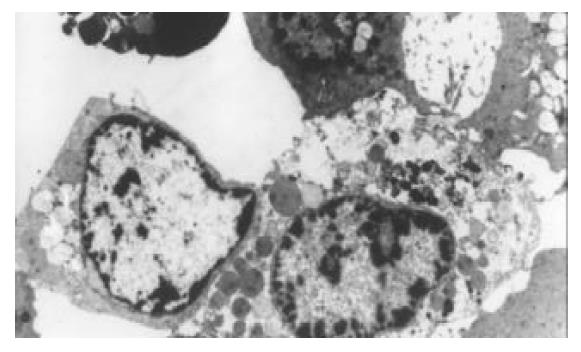
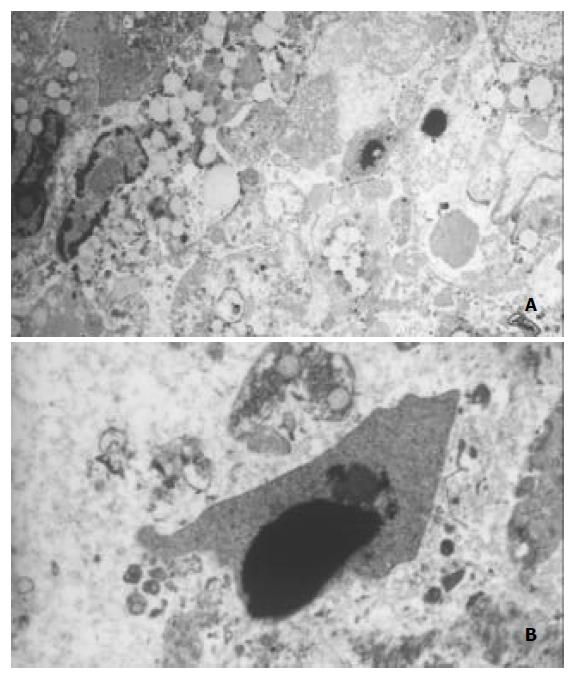
电镜下见细胞普遍脂滴较多, 可见细胞膜微绒毛消失, 边缘变平直, 细胞核皱缩, 染色质聚集在核模下等典型凋亡征象(图2A); 同时可见细胞器溶解、核碎裂等坏死征象(图2B).
FCM图像可见实验组细胞群坏死比例为30.7 %, 凋亡细胞占21.1 %. 而对照组仅有坏死细胞0.1 %, 凋亡细胞1.9 %. 结果说明凋亡相关过程在细胞死亡机制中起重要作用.
接种后10 d, 所有裸鼠均成瘤, 依分组情况分别给予处理. 处死裸鼠行瘤组织称重. SPLM软件行方差分析结果示3个实验组肿瘤较对照组均明显重量减轻(0.69±0.11, 1.22±0.22, 0.42±0.12 vs 2.26±0.23, P<0.01), 说明3个实验组均产生明显的抑瘤效果; 实验组3较实验组1 (P <0.05)、实验组2 (P <0.01)减轻更明显; 抑瘤率明显增加(81.5% vs 69.4%, 45.9 %) ; 实验组2较实验组1有显著差异(P <0.01), 说明实验组2的抑瘤作用较实验组1弱.
CD/5-FC系统是近年来研究较多的一种基因导向的酶/前体药物治疗(GDEPT)策略, 其应用于结直肠癌的研究目前已经进入I期临床阶段[10,11]. 5-FU作为结直肠癌的一线化疗药其全身给药毒副作用较大. CD/5-FC系统应用有效的载体使肿瘤组织特异性表达CD基因, 其编码的酶能将对哺乳动物本身相对无毒的5-FC转化为细胞毒性的5-FU, 从而表现出杀细胞效应.同时CD/5-FC系统还具有强大的旁观者效应. 另一方面, 胃泌素前体和甘胺酸胃泌素明显刺激结直肠癌细胞生长. 在过表达胃泌素前体和甘胺酸胃泌素的转基因鼠和结肠癌细胞系均能观察到这种作用. 胃泌素通过多种机制促进结肠癌细胞生长[12-14]. 多种胃泌素拮抗剂对结肠癌生长有轻度抑制作用已经得到证实[15]. CI-988抑制肠癌LoVo细胞生长已在体外培养和动物实验中得到证实. 本结果显示, 体外培养6 d时1 mmol/L 5-FC和1×10-8mmol/L CI-988分别可致转染了CD基因的LoVo细胞生长抑制率达90 %和50 %, 与文献报导一致, 二者联合使用杀伤作用更明显, 于培养3 d和6 d时抑瘤率达40 %和97 %. MTT法检测杀伤率, 联合应用1 mmol/L 5-FC和1×10-8 mmol/L CI-988较单用任何一种杀细胞作用更明显, 有统计学差异(P <0.05). 动物模型抑瘤实验结果证明, 单独使用 5-FC(500 mg/kg)腹腔注射或CI-988(10 mg/kg)灌胃均可产生明显抑瘤效果, 有统计学差异(P <0.01), 抑瘤率分别为69.4 %和45.9 %, 与文献报导一致, 联合应用二者抑瘤效果较单用任何一种措施更明显, 有统计学差异(P <0.05). 透射电镜下体外培养及动物模型中均可见兼有凋亡与坏死征象, 这种现象同时也在体外细胞培养的流式细胞仪检测中得到证实. 笔者前期实验已经证实CD/5-FC系统引起结肠癌LoVo细胞死亡的过程中有凋亡机制参与, 认为, 胃泌素拮抗剂可能通过提高结肠癌细胞对凋亡刺激的敏感性而与CD/5-FC产生协同治疗作用. 本结果再次验证了CD/5-FC系统和胃泌素拮抗剂CI-988对结肠癌在体外细胞培养和动物模型中的杀伤作用, 同时证明联合应用胃泌素拮抗剂CI-988可以提高CD/5-FC对结肠癌细胞的杀伤作用.
| 1. | Shen LZ, Wu WX, Xu DH, Zheng ZC, Liu XY, Ding Q, Hua YB, Yao K. Specific CEA-producing colorectal carcinoma cell killing with recombinant adenoviral vector containing cytosine deaminase gene. World J Gastroenterol. 2002;8:270-275. [DOI] |
| 2. | Nyati MK, Symon Z, Kievit E, Dornfeld KJ, Rynkiewicz SD, Ross BD, Rehemtulla A, Lawrence TS. The potential of 5-fluorocytosine/cytosine deaminase enzyme prodrug gene therapy in an intrahepatic colon cancer model. Gene Ther. 2002;9:844-849. [DOI] |
| 3. | Baque P, Pierrefite-Carle V, Gavelli A, Brossette N, Benchimol D, Bourgeon A, Staccini P, Saint-Paul MC, Rossi B. Naked DNA injection for liver metastases treatment in rats. Hepatology. 2002;35:1144-1152. [DOI] |
| 4. | Pierrefite-Carle V, Baque P, Gavelli A, Brossette N, Benchimol D, Bourgeon A, Saint Paul MC, Staccini P, Rossi B. Subcutaneous or intrahepatic injection of suicide gene modified tumour cells induces a systemic antitumour response in a metastatic model of colon carcinoma in rats. Gut. 2002;50:387-391. |
| 5. | Humphreys MJ, Ghaneh P, Greenhalf W, Campbell F, Clayton TM, Everett P, Huber BE, Richards CA, Ford MJ, Neoptolemos JP. Hepatic intra-arterial delivery of a retroviral vector expressing the cytosine deaminase gene, controlled by the CEA promoter and intraperitoneal treatment with 5-fluorocytosine suppresses growth of colorectal liver metastases. Gene Ther. 2001;8:1241-1247. |
| 6. | Siddheshwar RK, Gray JC, Kelly SB. Plasma levels of progastrin but not amidated gastrin or glycine extended gastrin are elevated in patients with colorectal carcinoma. Gut. 2001;48:47-52. [DOI] |
| 7. | Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology. 2000;119:162-171. |
| 8. | Kermorgant S, Lehy T. Glycine-extended gastrin promotes the invasiveness of human colon cancer cells. Biochem Biophys Res Commun. 2001;285:136-141. [DOI] |
| 9. | Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin (17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307-313. [DOI] |
| 10. | Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Hum Gene Ther. 2001;12:1594-1596. |
| 11. | Harvey BG, Maroni J, O'Donoghue KA, Chu KW, Muscat JC, Pippo AL, Wright CE, Hollmann C, Wisnivesky JP, Kessler PD. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13:15-63. [DOI] |
| 12. | Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin /TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest. 2000;106:533-539. |
| 13. | Hollande F, Choquet A, Blanc EM, Lee DJ, Bali JP, Baldwin GS. Involvement of phosphatidylinositol 3-kinase and mitogen-activated protein kinases in glycine-extended gastrin-induced dissociation and migration of gastric epithelial cells. J Biol Chem. 2001;276:40402-40410. [DOI] |
| 14. | Wu H, Rao GN, Dai B, Singh P. Autocrine gastrins in colon cancer cells Up-regulate cytochrome C oxidase Vb and down-regulate efflux of cytochrome c and activation of caspase-3. J Biol Chem. 2000;275:32491-32498. [DOI] |