修回日期: 2002-12-30
接受日期: 2003-01-08
在线出版日期: 2003-09-15
探讨bFGF是否通过PI3K/PKB途径调节p21WAF1的表达.
32P掺入法检测PKB酶活性, RT-PCR、Western blot检测不同处理组Bel-7402细胞的p21WAF1表达, 流式细胞术分析细胞周期.
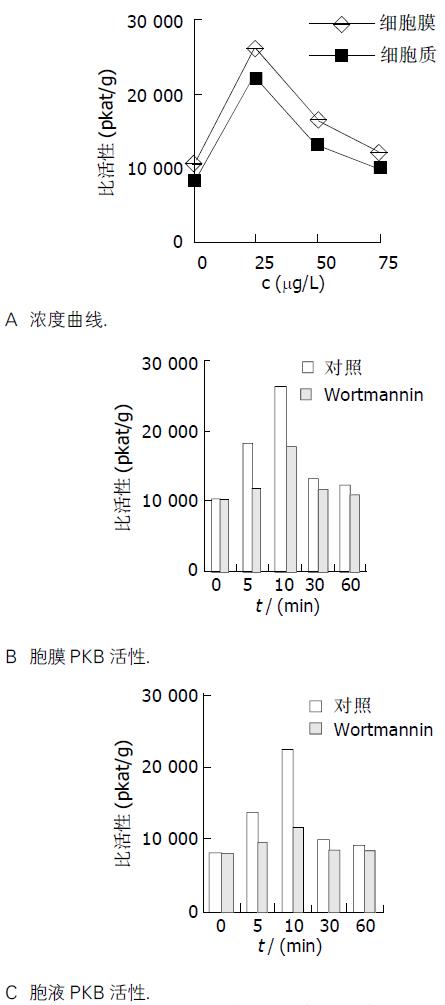
25 mg/L bFGF刺激细胞10 min, 就可使胞液和膜性PKB酶活性达高峰. p21WAF1mRNA表达水平在1 h达高峰, 比对照升高了5.5倍. p21WAF1蛋白表达在2 h达高峰, 比对照升高了2.2倍. wortmannin预处理后, PKB活性在各时间点均明显降低(P <0.05), p21WAF1 mRNA表达及p21WAF1蛋白表达无明显变化. 流式细胞术分析显示, bFGF处理组与对照组相比G1期细胞减少(0.65±0.01→0.49±0.02, P <0.01), S期细胞增多(0.14±0.01→0.28±0.01, P <0.01), wortmannin能抑制此促增生作用(G1: 0.58±0.01; S: 0.22±0.01, P <0.01).
PI3K/PKB途径可介导bFGF对Bel-7402细胞的促增生作用, 但是bFGF对p21WAF1表达的调节作用不依赖PI3K/PKB途径.
引文著录: 于卉影, 孙利平, 孙黎光, 丁晓慧. bFGF对人肝癌细胞系Bel-7402的生长调控. 世界华人消化杂志 2003; 11(9): 1333-1336
Revised: December 30, 2002
Accepted: January 8, 2003
Published online: September 15, 2003
To investigate whether bFGF regulates p21WAF1 expression of Bel-7402 cell line via PI3K/PKB pathway.
32P incorporation assay. The expression of p21WAF1-mRNA was assessed by RT-PCR. The expression of p21WAF1 protein was detected by Western blot. Cell cycle analysis was performed on a FACScan.
Both membrane and cytosol activity of PKB in Bel-7402 cell which were treated with bFGF (25 mg/L) reached the peak at 10 min. p21WAF1 mRNA level was upregulated and peaked at 1 h (5.5 fold induction). Correspondingly, p21WAF1 expression was increased and peaked at 2 h (2.2 folds of reduction). Wortmannin efficiently inhibited the activity of PKB (P <0.05), but not the level of p21WAF1 mRNA and the expression of p21WAF1 protein. FCM analysis showed bFGF induced S-phase entry (0.14±0.01→0.28±0.01, P <0.01), which was inhibited by wortmannin effectively (0.28±0.01→0.22±0.01, P <0.01).
bFGF stimulates the proliferation of Bel-7402 cell line via PI3K/PKB pathway, and modulates p21WAF1 expression through separating signaling pathways.
- Citation: Yu HY, Sun LP, Sun LG, Ding XH. Effect of basic fibroblast growth factor on growth regulation in Bel-7402 cell line. Shijie Huaren Xiaohua Zazhi 2003; 11(9): 1333-1336
- URL: https://www.wjgnet.com/1009-3079/full/v11/i9/1333.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i9.1333
成纤维细胞生长因子(FGFs)和成纤维细胞生长因子受体的信号转导通路与肿瘤发生发展的关系极为密切, 是细胞信号转导研究的热点[1-20]. PI3K/PKB是近年来发现的一个新的生长因子信号转导途径. 越来越多的证据表明, PKB与细胞代谢, 生长、凋亡、恶变等密切相关[21-29]. p21WAF1是目前已知的具有最广泛激酶抑制活性的细胞周期抑制蛋白[30-32], 他通过依赖和非依赖P53途径, 对细胞内信号和细胞外信号均能作出反应, 参与细胞生长、发育、分化、衰老及DNA损伤修复等多种功能的调节[33-35]. 我们采用外源性bFGF直接作用于Bel-7402肝癌细胞, 观察其对p21WAF1表达的影响, 探讨生长因子通过PKB途径调控细胞周期进程的信号转导机制.
人肝癌细胞株Bel-7402购自是中科学院上海细胞所, DMEM培养基为Gibcolbrl 公司产品, 小牛血清购自华美生物工程公司, TRIzol为Life-Technology公司产品, 扶济复(重组人bFGF)购自北京双鹭药业有限公司, 组蛋白2B为Roche 公司产品, cAMP蛋白激酶抑制剂为Sigma 公司产品, [γ-32P] ATP购于北京福瑞生物工程公司, 单克隆鼠抗人p21WAF1、辣根过氧化物酶标记的二抗和ECL试剂盒购于北京中山生物技术公司, TaKaRa RNA PCR 试剂盒购自大连宝生物工程有限公司, 其余试剂均为国产分析纯试剂.
1.2.1 32P掺入法检测PKB酶活性 将对数生长期细胞1×108/L, 接种于培养瓶, 待达到70 %融合时换成无血清DMEM培养液孵育过夜, 使细胞同步化, 然后随机按下述时间点和浓度点分组施加因素. 分组如下: (1)不同剂量组: 0, 25, 50, 75 mg/L, 处理时间10 min; (2)不同时间组: 0, 5, 10, 60 min, 剂量为25 mg/L; (3)wortmannin预处理组: 处理时细胞同步化同前, 空白对照以等量二甲基亚砜替代wortmannin, 加入wortmannin 终浓度100 nmol/L, 置于细胞培养箱中孵育1 h, 然后按(2)的各时间点加bFGF 处理. 处理期间细胞置于细胞培养箱中. 处理结束后, 收集细胞, 用冰PBS洗涤3次. 按照文献[36]方法进行PKB酶活性检测.
1.2.2 Western blot 分析 取各组细胞40 mg总蛋白上样于150 g/L SDS-PAGE电泳分离, 电转移至硝酸纤维素膜上, 加入一抗, 孵育2 h, 洗膜, 加入羊抗鼠辣根过氧化物酶标二抗, 孵育2 h, 按ECL免疫印迹检测试剂盒说明进行显色、曝光. 分组如下: (1)bFGF组: 0, 30 min, 1 h, 2 h, 6 h, 剂量为25 mg/L; (2)wortmannin+bFGF组: 先加入100 nmol/L wortmannin, 孵育1 h, 然后按(1)的各时间点加bFGF 处理.
1.2.3 RT-PCR检测p21WAF1mRNA 表达 应用premer Primer5软件设计引物, 由大连宝生物工程有限公司合成, p21WAF1:上游引物5'-ACTTTGTCACCGAGACACCA-3'; 下游引物5'-CCTCAGCCTTCCAAGTAGC-5'. b-actin: 上游引物 5'-GATGACCCAGATCATGTTTG-3';下游引物 5'-TGGAGTTGAAGGTAGTTTCG-3'. 按Trizol试剂操作说明提取各处理组细胞的总RNA. 紫外检测仪分别在260 nm和280 nm波长下检测RNA的吸光度A值, 测定RNA的纯度. cDNA合成后, 建立25 mL反应体系, 95 °C预变性90 s, 循环28次, 循环条件为: 94 °C变性1 min, 58 °C退火1 min, 72 °C延伸1 min. 循环结束后72 °C延伸10 min. PCR产物用15 g/L琼脂糖凝胶电泳, 凝胶成像仪扫描摄影. 分组如下: (1) bFGF组: 0, 30 min, 1 h, 2 h, 剂量为25 mg/L; (2) wortmannin+bFGF组: 先加入100 nmol/L wortmannin, 孵育1 h, 然后按(1)的各时间点加bFGF 处理.
1.2.4 细胞周期分析 收集消化的贴壁细胞, 离心后重悬在含100 mg/L RNA酶和10 g/L碘化丙啶的PBS中, 用FACScan 检测, 结果用Cell-Quest软件分析数据.
统计学处理 差异显著性采用t 检验.
采用外源性bFGF刺激细胞, 检测PKB酶活性, 计算比活性值绘制浓度曲线(图1A)和时间曲线(图1B, C). 结果发现, 不同浓度的bFGF孵育Bel-7402细胞迅速激活PKB, 且膜性PKB活性高于胞液PKB活性. 25 mg/L bFGF刺激细胞10 min, 就可使胞液和膜性PKB酶活性达高峰.
经25 mg/L bFGF处理后, Bel-7402细胞p21WAF1mRNA表达水平增高 (见图2), 在1 h达高峰, 与对照相比升高了5.5倍. Western blot 分析显示, 经25 mg/L bFGF处理后, Bel-7402细胞的p21WAF1蛋白表达增加, 在2 h达高峰, 与对照相比升高了2.2倍 (图3).
经PI3K抑制剂wortmannin预处理与未经预处理的各组相比, PKB活性在各时间点均存在显著差异(P <0.05), p21WAF1 mRNA表达及p21WAF1蛋白表达均无明显变化.
FCM显示, 经25 mg/L bFGF孵育16 h后,与对照组相比G1期细胞减少(0.65±0.01→0.49±0.02, P<0.01), S期细胞增多(0.14±0.01→0.28±0.01, P <0.01), 但wortmannin能抑制此促增生作用(G1: 0.58±0.01; S: 0.22±0.01, P <0.01).
大量研究表明, FGFS/FGFRS信号传递与肿瘤发生发展的关系极为密切, 多种肿瘤细胞表达水平极高的FGFR1和aFGF/bFGF, 以过量表达FGFR1和aFGF/bFGF为特征的自分泌信号环路是肿瘤细胞恶性增生的首要条件. 研究肿瘤细胞信号传导机制, 选择性的阻断肿瘤细胞自分泌或旁分泌的信号传导通路, 破坏其自控性生长调节机制, 正在成为极具吸引力的研究热点[33]. p21 WAF1是最早发现并克隆的周期素依赖性激酶抑制剂, 对细胞内信号和细胞外信号均能作出反应, 参加细胞多种功能活动 [30,34]. p21WAF1通过抑制Cyclin-CDK复合物活性, 充当协调细胞周期转换、检查点控制的内部定时机制, 而外部生长控制信号通过影响p21WAF1功能来控制细胞周期[31,35]. bFGF作用于MCF-7细胞, 既能增加p21WAF1的转录和表达并使cdk2失活及Rb去磷酸化, 降低cyclin A的表达, 同时又上调G1期蛋白如cyclin D1、cyclin E和cdk4的表达, 此双重作用的结果是抑制MCF-7细胞的增生. FGF通过不依赖MAPK的途径活化STAT1, 上调p21WAF1的mRNA水平和蛋白表达, 抑制MCF-7细胞增生[18]. FGF-2处理NIH3T3细胞后, 通过非依赖MAPK途径诱导p21WAF1蛋白表达, 但FGF-2对p21WAF1mRNA水平的上调作用部分依赖MAPK途径, 并且通过MAPK途径促进细胞进入S期[19]. 我们采用外源性bFGF处理Bel-7402细胞, 结果表明bFGF能促进p21WAF1 mRNA水平升高, 导致p21WAF1的表达增加. PI3K抑制剂wortmannin预处理的细胞, 经bFGF刺激后, 在各时间点p21WAF1 mRNA水平无明显改变, 同时p21WAF1表达水平也无明显变化, 表明bFGF对Bel-7402 p21WAF1表达的影响是通过影响其转录活性实现的, 但是这种调节作用不依赖PI3K/PKB途径. FCM检测细胞周期结果表明, bFGF处理后细胞G1期比例下降, S期比例增加, 这与bFGF促进细胞增生相符, 预先加入PI3K 抑制剂wortmannin再用 bFGF处理后, 这种促增生作用受到抑制(P <0.01), 提示bFGF通过PI3K/PKB途径促进细胞周期进程, 使细胞从G1期→S期. 可见, bFGF对p21WAF1表达的诱导作用和对细胞周期进程的促进作用是通过不同途径实现的. 已知p21WAF1是具有最广泛激酶抑制活性的细胞周期抑制蛋白, 可与几乎每一个Cyclin-CDK复合物结合. p21WAF1抑制Cyclin D-CDK4和Cyclin E-CDK2的活性, 使Rb蛋白不能磷酸化, 从而使细胞周期停止在G1期. p21WAF1通过与Cyclin A-CDK2结合间接抑制DNA合成[32-36]. 本结果表明bFGF既能上调p21WAF1的表达, 同时又促进细胞进入S期, 这似乎是矛盾的. 但是, 这两种作用是通过不同途径发生的. bFGF能调节周期素和周期素依赖性激酶的表达[17]. 因此, 我们可以初步推测如果bFGF诱导过量Cyclin/CDK的复合物形成, 超过了p21WAF1的结合能力, 细胞将从G1期→S期.
| 1. | Zimering MB, Thakker-Varia S. Increased fibroblast growth factor-like autoantibodies in serum from a subset of patients with cancer-associated hypercalcemia. Life Sci. 2002;71:2939-2959. [DOI] |
| 2. | Keyes K, Cox K, Treadway P, Mann L, Shih C, Faul MM, Teicher BA. An in vitro tumor model: analysis of angiogenic factor expression after chemotherapy. Cancer Res. 2002;62:5597-5602. |
| 3. | Hu M, Nicolson GL, Trent JC 2nd, Yu D, Zhang L, Lang A, Killary A, Ellis LM, Bucana CD, Pollock RE. Characterization of 11 human sarcoma cell strains: evaluation of cytogenetics, tumorigenicity, metastasis, and production of angiogenic factors. Cancer. 2002;95:1569-1576. [DOI] |
| 4. | Joensuu H, Anttonen A, Eriksson M, Makitaro R, Alfthan H, Kinnula V, Leppa S. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res. 2002;62:5210-5217. |
| 5. | Coleman AB, Metz MZ, Donohue CA, Schwarz RE, Kane SE. Chemosensitization by fibroblast growth factor-2 is not dependent upon proliferation, S-phase accumulation, or p53 status. Biochem Pharmacol. 2002;64:1111-1123. [DOI] |
| 6. | Jeffers M, LaRochelle WJ, Lichenstein HS. Fibroblast growth factors in cancer: therapeutic possibilities. Expert Opin Ther Targets. 2002;6:469-482. [DOI] |
| 7. | Adriaenssens E, Lemoine J, El Yazidi-Belkoura I, Hondermarck H. Growth signaling in breast cancer cells: outcomes and promises of proteomics. Biochem Pharmacol. 2002;64:797-803. |
| 8. | Dini G, Funghini S, Witort E, Magnelli L, Fanti E, Rifkin DB, Del Rosso M. Overexpression of the 18 kDa and 22/24 kDa FGF-2 isoforms results in differential drug resistance and amplification potential. J Cell Physiol. 2002;193:64-72. |
| 9. | Glenjen N, Mosevoll KA, Bruserud O. Serum levels of angiogenin, basic fibroblast growth factor and endostatin in patients receiving intensive chemotherapy for acute myelogenous leukemia. Int J Cancer. 2002;101:86-94. [DOI] |
| 10. | Song Z, Wu X, Powell WC, Cardiff RD, Cohen MB, Tin RT, Matusik RJ, Miller GJ, Roy-Burman P. Fibroblast growth factor 8 isoform B overexpression in prostate epithelium: a new mouse model for prostatic intraepithelial neoplasia. Cancer Res. 2002;62:5096-5105. |
| 11. | Papanikolaou IS, Lazaris AC, Kavantzas N, Davaris PS. Minimal expression of the proto-oncogene int-2 encoded protein in a series of colorectal carcinomas. J Gastroenterol Hepatol. 2002;17:1084-1086. [DOI] |
| 12. | Pavlakovic H, Havers W, Schweigerer L. Multiple angiogenesis stimulators in a single malignancy: implications for anti-angiogenic tumour therapy. Angiogenesis. 2001;4:259-262. |
| 13. | Berglund A, Molin D, Larsson A, Einarsson R, Glimelius B. Tumour markers as early predictors of response to chemotherapy in advanced colorectal carcinoma. Ann Oncol. 2002;13:1430-1437. [DOI] |
| 14. | Deniz ML, Kilic T, Almaata I, Kurtkaya O, Sav A, Pamir MN. Expression of growth factors and structural proteins in chordomas: basic fibroblast growth factor, transforming growth factor alpha, and fibronectin are correlated with recurrence. Neurosurgery. 2002;51:753-760. |
| 15. | Ngan ES, Ma ZQ, Chua SS, DeMayo FJ, Tsai SY. Inducible expression of FGF-3 in mouse mammary gland. Proc Natl Acad Sci USA. 2002;99:11187-11192. |
| 16. | Koh KR, Ohta K, Nakamae H, Hino M, Yamane T, Takubo T, Tatsumi N. Differential effects of fibroblast growth factor-4, epidermal growth factor and transforming growth factor-beta1 on functional development of stromal layers in acute myeloid leukemia. Leuk Res. 2002;26:933-938. [DOI] |
| 17. | Wang HS, Mark R, Eyal F, Anthony D, John M, Joachim Y, Robert W. Basic fibroblast grown factor causes grown arrest in MCF-7 human breast cancer cells while inducing both mitogenic and inhibitory G1 evants. Cancer Res. 1997;57:1750-1757. |
| 18. | Johnson MR, Valentine C, Basilico C, Mansukhani A. FGF signaling activates STAT1 and p21 and inhibits the estrogen response and proliferation of MCF-7 cells. Oncogene. 1998;16:2647-2656. |
| 19. | Kivinen L, Laiho M. Ras-and mitogen-activated protein kinase kinase-dependent and 杋ndependent pathways in p21Cip1/WAF1 induction by fibroblast growth factor-2, platelet-derived growth factor, and transforming growth factor-beta1. Cell Growth Differ. 1999;10:621-628. |
| 20. | Fu XB, Yang YH, Sun TZ, Gu XM, Jiang LX, Sun XQ, Sheng ZY. Effect of intestinal ischemia-reperfusion on expressions of endogenous basic fibroblast growth factor and transforming growth factor b in lung and its relation with lung repair. World J Gastroenterol. 2000;6:353-355. [DOI] |
| 21. | Al-Sakkaf KA, Mooney LM, Dobson PR, Brown BL. Possible role for protein kinase B in the anti-apoptotic effect of prolactin in rat Nb2 lymphoma cells. J Endocrinol. 2000;167:85-92. [DOI] |
| 22. | Demoulin JP, Grasso L, Atkins JM, Stevens M, Louahed J, Levitt RC, Nicolaides NC, Renauld JC. Role of insulin receptor substrate-2 in interleukin-9-dependent proliferation. FEBS Lett. 2000;482:200-204. |
| 23. | Murao K, Ohyama T, Imachi H, Ishida T, Cao WM, Namihira H, Sato M, Wong NC, Takahara J. TNF-alpha stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochem Biophys Res Commun. 2000;276:791-796. |
| 24. | Hixon ML, Muro-Cacho C, Wagner MW, Obejero-Paz C, Millie E, Fujio Y, Kureishi Y, Hassold T, Walsh K, Gualberto A. Akt1/PKB upregulation leads to vascular smooth muscle cell hypertrophy and polyploidization. J Clin Invest. 2000;106:1011-1120. |
| 25. | Iynedjian PB, Roth RA, Fleischmann M, Gjinovci A. Activation of protein kinase B/cAkt in hepatocytes is sufficient for the induction of expression of the gene encoding glucokinase. Biochem J. 2000;351:621-627. |
| 26. | Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991-6000. [DOI] |
| 27. | Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7:3269-3275. |
| 28. | Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002;107:80-88. [DOI] |
| 29. | Lorenzini A, Tresini M, Mawal-Dewan M, Frisoni L, Zhang H, Allen RG, Sell C, Cristofalo VJ. Role of the Raf/MEK/ERK and the PI3K/Akt(PKB) pathways in fibroblast senescence. Exp Gerontol. 2002;37:1149-1156. [DOI] |
| 30. | Boulaire J, Fotedar A, Fotedar R. The functions of the cdk-cyclin kinase inhibitor p21WAF1. Pathol Biol. 2000;48:190-202. |
| 31. | Fang JY, Lu YY. Effects of histone acetylation and DNA methylation on p21( WAF1) regulation. World J Gastroenterol. 2002;8:400-405. |
| 32. | Sun BH, Zhang J, Wang BJ, Zhao XP, Wang YK, Yu ZQ, Yang DL, Hao LJ. Analysis of in vivo patterns of caspase 3 gene expression in primary hepatocellular carcinoma and its relationship to p21(WAF1) expression and hepatic apoptosis. World J Gastroenterol. 2000;6:356-360. [DOI] |
| 33. | Yin F, Yao SK, Wu XM, Gao HS, Li ZH. Serapharmacological study of Ganzheng oral solution on proliferation and expression of ERK in SMMC-7721 cells with transforming growth factor a. Shijie Huaren Xiaohua Zazhi. 2001;9:1017-1020. |
| 34. | Hu PZ, Zhang CS, Ma FC, Yang SJ, Wang WL. Expressions of cyclin- dependent kinase inhibitor P21WAF1/CIP1 and PCNA in human hepatocellular c arcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:145-148. |
| 35. | Liu J, Chen SL, Zhang W, Su Q. P21WAF1 gene expression with P53 mutation in esophageal carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:1350-1353. |
| 36. | Liu SL, Liu GZ, Cheng J, Shi DY, Chen HL, Zhang YD. Influence of PKB on ROS regulation of proliferation in human 7721 hepatoma cells. Acta Biochimicaet Biophysica Sinica. 2002;34:67-72. |