修回日期: 2002-05-20
接受日期: 2002-06-26
在线出版日期: 2003-07-15
观察蜂毒素-聚乳酸/羟乙酸微球(M-MS)经动脉给药对大鼠肝肿瘤的疗效.
采用改良的复乳-液中蒸发法制备蜂毒素-聚乳酸/羟乙酸微球, 建立大鼠移植性肝癌模型并随机分为对照组, 蜂毒素组, 空白微球组和蜂毒素微球组, 每组16只. 分别经肝动脉注入生理盐水(NS, 1.5mL/kg)、蜂毒素(Melittin, 0.35 mg/kg)、空白聚乳酸/羟乙酸微球(B-MS, 10 mg/kg)和蜂毒素-聚乳酸/羟乙酸微球(M-MS, 10 mg/kg). 比较治疗后各组大鼠的肿瘤生长情况、肿瘤坏死程度和生存时间.
治疗后, 与对照组比较, 蜂毒素组和空白微球组肿瘤生长率均显著降低(12.4±7.1, 10.1±8.2 vs 28.3±13.6, P<0.01), 肿瘤坏死程度以轻中度为主, 但两组动物生存时间均未能明显延长(15.8±2.0 d, 16.5±3.0 d vs 13.7±2.2 d , P>0.05). M-MS组肿瘤生长率(1.1±1.1)明显低于其他3组(P<0.01), 肿瘤坏死更广泛, 更彻底, 且与对照组比较经蜂毒素微球治疗的大鼠生存期(31.0±3.9 d)显著延长(P<0.01).
蜂毒素以药物微球的剂型经肝动脉给药, 抗肿瘤效果明显优于单纯的蜂毒素和空白微球.
引文著录: 凌昌全, 李琦, 刘晓华, 陈庆华, 彭永海, 罗若茵, 黄雪强. 经动脉灌注蜂毒素-聚乳酸/羟乙酸微球治疗大鼠肝肿瘤. 世界华人消化杂志 2003; 11(7): 900-903
Revised: May 20, 2002
Accepted: June 26, 2002
Published online: July 15, 2003
To observe the therapeutic effects of poly lactic-co-glycolic acid (PLGA) microspheres containing Melittin (M-MS) infused via artery on hepatocarcinoma in rats.
M-MS was prepared with biodegradable poly lactic-co-glycolic acid by multiple emulsions in liquid evaporation process. Rats bearing transplanted hepatoma were established and were randomly divided into control group, melittin group, blank microsphere (B-MS) and M-MS group with 16 rats in each group, in each respective group, normal saline (NS, 1.5 mL/kg), melittin (0.35 mg/kg), blank microspheres (10 mg/kg), and M-MS(10 mg/kg) were infused via gastro duodenal artery into hepatic artery. The tumor growth, severity of necrosis and survival of rats were documented.
Compared with control group, the tumor growth in melittin and B-MS groups were significantly inhibited (12.4±7.1, 10.1±8.2 vs 28.3±13.6, P<0.01) and the tumor necrosis degree were dominantly in low- and moderate-grade, but the survival were not prolonged obviously (15.8±2.0 d, 16.5±3.0 d vs 13.7±2.2, P>0.05). Meanwhile, the tumor growth in M-MS group (1.11.1) was much slower than that of other 3 groups and tumor necrosis degree were mainly in severe and the survival (31.03.9 d) of rats was also significantly prolonged (P<0.01).
The anti-tumor effect of M-MS administered via hepatic artery is much higher than that of melittin and B-MS.
- Citation: Ling CQ, Li Q, Liu XH, Chen QH, Peng YH, Luo RY, Huang XQ. Infusion of Melittin-poly lactic-co-glycolic acid microspheres via hepatic artery for hepatocarcinoma in rats. Shijie Huaren Xiaohua Zazhi 2003; 11(7): 900-903
- URL: https://www.wjgnet.com/1009-3079/full/v11/i7/900.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i7.900
目前肝癌的手术切除率仅有20%[1], 对于不能手术切除的肝癌, 肝动脉化疗栓塞(TACE)是首选的治疗方法[2-12]. 药物微球作为一种新剂型通过肝动脉介入, 可栓塞至肝动脉末梢, 有效的阻断肝癌的营养来源, 而且在肿瘤局部缓释其包裹的药物, 具有控释、末梢栓塞、靶向等多重作用, 能够提高药物肝动脉介入的抗肿瘤效果[13-15]. 我们参照文献[16], 采用改良的复乳-液中干燥法制备蜂毒素-聚乳酸/羟乙酸微球, 经肝动脉灌注治疗大鼠移植性肝癌, 取得了初步的研究结果, 现报道如下.
蜂毒素(melittin)由第二军医大学长海医院中西医结合肿瘤实验室从中华蜜蜂毒中分离、纯化(纯度>95%). 蜂毒素-聚乳酸/羟乙酸微球(M-MS)、聚乳酸/羟乙酸空白微球(B-MS)由上海医药工业研究院采用改良的复乳-液中蒸发法制备, 微球粒径均为40-100 um, M-MS载药量为3.5%. 微球经1.0×106 Gy 剂量的60Co照射灭菌, 照射后经检测其形态、蜂毒素生物活性、含量等无改变. 体外溶出试验: 首日释药15%, 以后以2.6%/d的速度持续释放5 wk以上. 双人双目手术显微镜、显微外科手术包购自苏州医疗器械总厂. ♂SD大鼠, 质量200-250 g, 购自第二军医大学实验动物中心. 大鼠Walker-256瘤株由上海长海医院上海市中西医结合肿瘤实验室提供. 大鼠移植性肝癌模型的制作参照Trubenbach et al[17]的方法 而有所改进: Walker-256瘤株从液氮中复苏后培养扩增, 调整细胞悬液浓度至1×106/L细胞, 注入质量为100 g左右SD幼鼠腹腔1 mL. 1 wk后幼鼠出现腹水, 抽取腹水, 以1000 r/min的速度离心3 min, 生理盐水洗涤2遍后再离心, 吸弃上清, 得糊状细胞匀浆, 备用. SD大鼠术前禁食12 h, 用20 g/L戊巴比妥钠40 mg/kg剂量腹腔注射麻醉, 仰卧位固定于手术板上, 术区常规消毒, 无菌条件下, 在剑突下, 沿腹白线切开1.5 cm左右, 暴露肝脏, 托出肝左外叶, 用1 mL一次性注射器抽取细胞匀浆, 针头与肝脏成300角斜刺入肝脏0.5 cm, 注入细胞匀浆0.05 mL, 拔除针头, 棉签压迫, 检查无渗血后关腹.
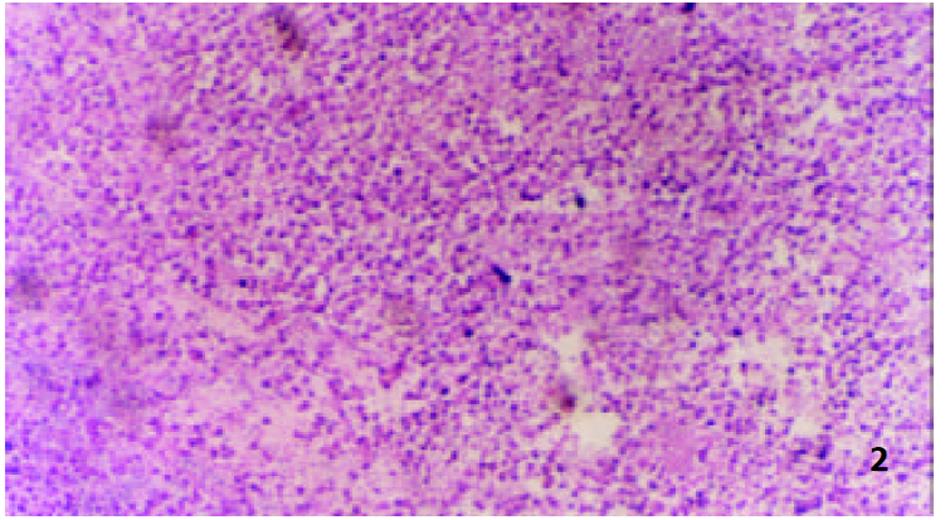
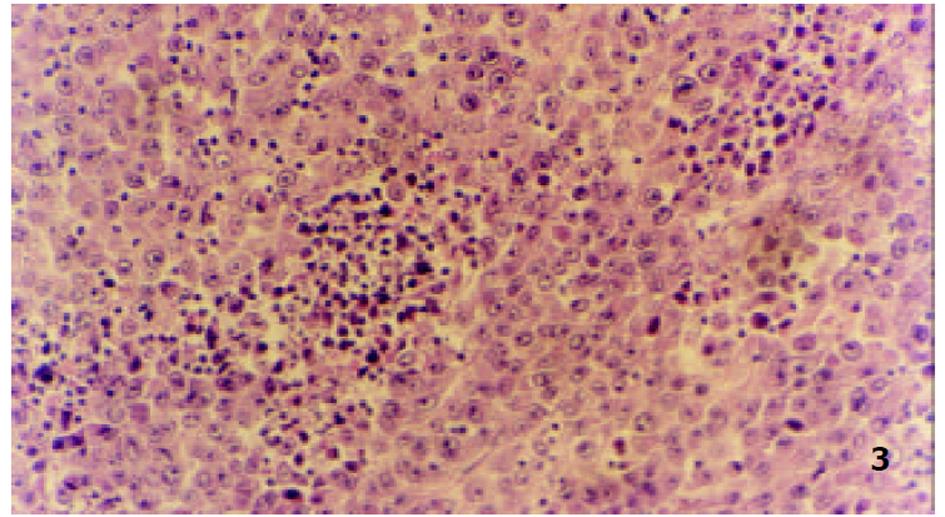
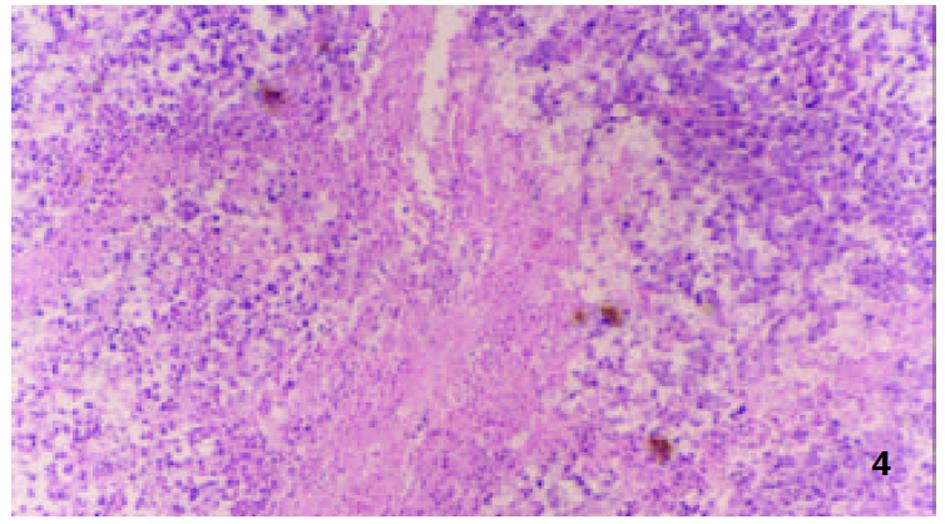
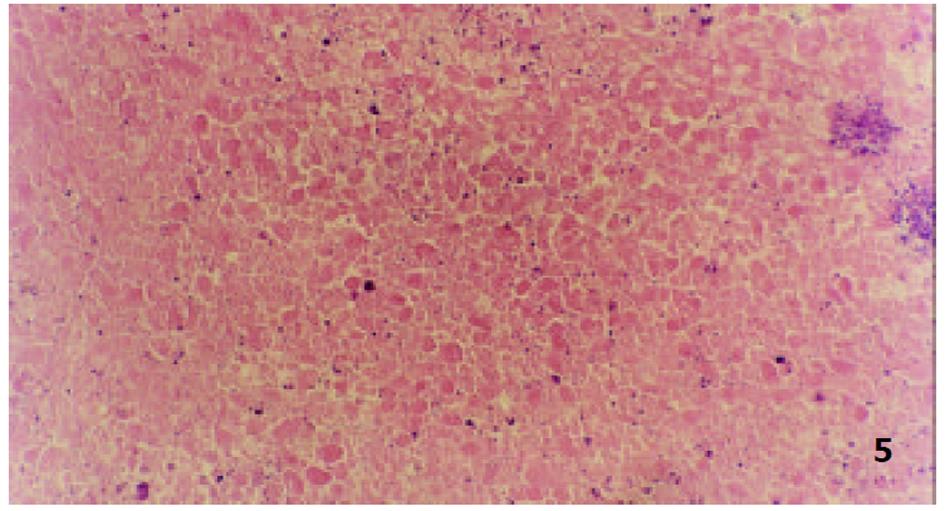
参照Lindell et al(Eur Surg Res 1988; 9: 347-352)的肝动脉插管方法, 大鼠造模后7 d, 无菌下再次剖腹, 暴露肝脏. 在手术显微镜下: 用游标卡尺测定肿瘤最大长径和最短径; 分离胃十二指肠动脉、肝总动脉和肝固有动脉. 结扎胃、十二指肠动脉远端, 血管夹暂时阻断肝总动脉, 在胃、十二指肠动脉上作一小切口, 由此插入外径为0.4 mm的自制硅胶导管, 上行至肝固有动脉, 用9-0缝合线固定, 回血后, 分别缓慢注入不同制剂, 拔出导管, 结扎胃十二指肠动脉近端, 放开肝总动脉上的血管夹, 关腹分笼饲养. 随机分为对照组、melittin组、B-MS组和M-MS组, 每组16只. 分别经肝动脉灌注生理盐水1.5 mL/kg, melittin 0.35 mg/kg, B-MS 10 mg/kg, M-MS10 mg/kg. 后3组药物均溶于生理盐水, 大鼠肝动脉灌注体积均为1.5 mg/kg. 各组随机取6只大鼠于治疗后次日起观察生存天数, 并以生理盐水组为对照组计算生命延长率(%). 生命延长率(%) = (治疗组平均存活天数/对照组平均存活天数-1)×100%. 各组余下动物于治疗后8 d处死, 测量肿瘤长径(a)和短径(b), 按公式V = ab2/2[18]计算肿瘤体积, 再根据治疗前后的肿瘤体积比计算肿瘤生长率(growth rates, GR)[19,20]. GR = 治疗后的肿瘤体积/治疗前的肿瘤体积. 完整切取以上处死的大鼠瘤块, 置40 g/L甲醛液中固定, 常规石蜡包埋, 取瘤体最大剖面作2-3处病理切片, HE染色, 光镜下, 根据坏死组织所占整个瘤体的比例分为3度: 轻度(0-30%)、中度(31-70%)及重度(71-100%), 比较各组肿瘤坏死程度.
统计学处理 运用SPSS10.0统计软件, 采用student t, rank sum test, SNK检验方法进行统计分析. P<0.05具有显著性差异.
由于手术操作损伤血管, 4组64只大鼠术中及术后有5只出血死亡. 治疗前各组肿瘤体积无显著性差异(P>0.05)具有可比性. 对照组治疗后肿瘤体积显著增大(P<0.01), 部分大鼠出现肿瘤侵袭腹壁、胃等周围组织及腹水形成; Melittin组和B-MS组肿瘤体积较治疗前也明显增大(P<0.01), 两组肿瘤生长率均低于对照组(P<0.01); M-MS组肿瘤体积与治疗前相比无明显改变(P>0.05), 其中治疗后1例肿瘤消失, 1例无明显改变, 4例缩小, 其余4例均有不同程度的增大, 肿瘤生长率明显低于其他3组(P<0.01, 表1, 图1).
治疗后各组荷瘤大鼠平均生存时间有显著差异(F = 46.0626, P<0.01, 表2). M-MS组与对照组、B-MS、Melittin组相比, 生存时间明显延长(P<0.01). 经B-MS、Melittin治疗的荷瘤大鼠生存时间虽长于对照组, 但其差异均无统计学意义.
对不能手术切除的肝癌, 肝动脉化疗栓塞(TACE)是首选的治疗方法. 临床上常用的栓塞剂碘油, 作为药物载体虽然可选择性滞留在肿瘤组织, 具有缓释性, 但碘油只能到达较细的血管分支, 无法栓塞微血管网[1], 且碘油与化疗药相溶性差, 药物释放不稳定; 明胶海绵仅能栓塞至肝动脉2-3级分枝, 栓塞后, 侧支循环容易建立[21], 以及常规TACE存在的毒副反应[22,23], 致使TACE近期疗效显著显著, 远期疗效并不理想[24-28]. 提高肝癌介入的效果, 除改进导管操作技术外, 研制栓塞、抗癌、低毒高效的介入新制剂, 是该领域研究的重要发展方向之一. 药物微球是近二十年来研制的一种新剂型, 由于微球粒径可以按照人为的需要制备, 通过肝动脉栓塞能栓塞至肝窦前小动脉水平, 与其他栓塞剂相比栓塞更彻底, 侧枝循环不易形成, 所以能很好的切断肿瘤的营养来源. 在发挥栓塞作用的同时, 微球内药物可在肝癌局部持续缓慢释放, 使局部保持较高的浓度, 起到良好的被动靶向抗癌作用, 能显著降低系统毒性, 具有控释、末梢栓塞、靶向的多重功效[29-32]. 业已证实药物微球具有抗癌效果好, 使用方便, 用量小, 末梢栓塞作用强等优点.
蜂毒素为蜜蜂毒液中的一种多肽, 由26个氨基酸组成, 以往临床上主要用于治疗自身免疫性疾病. 但近20 a来, 先后发现对多种实验性肿瘤有强烈的杀灭作用[33,34], 引起了人们的极大关注. 蜂毒素能使肿瘤细胞微粒体膜溶解, 使其呼吸受到抑制, 从而发挥抗肿瘤作用. 但蜂毒素具有溶血等毒副作用, 限制了在临床上的应用, 至今未见有用于治疗肿瘤的临床报道. 我们在体外细胞试验和局部注射治疗肿瘤取得了肯定疗效的基础上, 将蜂毒素以药物微球的方式, 经肝动脉介入给药, 药物在肿瘤局部缓慢释放, 局部药物浓度高, 全身浓度低, 起到了很好的减毒增效作用. 蜂毒素微球在抑制肿瘤生长, 延长荷瘤大鼠生存时间方面明显的优于蜂毒素和空白微球. 一方面是蜂毒素微球通过选择性栓塞肿瘤血管引起肿瘤缺血、缺氧坏死, 另一方面蜂毒素从微球中缓慢释放, 局部药物浓度高, 对肿瘤组织的作用时间长, 缺氧状态的癌细胞对药物更敏感, 使治疗效果增强. 单纯蜂毒素动脉灌注, 虽然也能提高局部药物浓度, 但由于肝脏存在门静脉、肝动脉双重血供, 血流量大, 经肝动脉灌注的药物易被血流冲洗, 使局部停留时间短, 故单纯蜂毒素动脉灌注, 抗癌效果不理想. 另外, 实验中未发现溶血等毒副作用, 可能与药物主要分布在肿瘤组织, 全身浓度较低有关. 我们以聚乳酸羟乙酸作为载体, 制成的蜂毒素-聚乳酸/羟乙酸微球, 国内外尚未见报道. 我们的研究结果表明, 将蜂毒素以微球的剂型肝动脉给药治疗肝癌是可行的, 而且能够达到"祛邪而不伤正"的目的. 有关蜂毒素-聚乳酸/羟乙酸微球的抗癌确切机制尚有待于进一步研究.
| 1. | Qi YY, Zou LG, Bian XW. Angiogenesis and transcather arterial chemoembolization of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:960-965. |
| 2. | Gao ZE, Zhang CX, Pang MX, Sun P. Charaterics of blood donation in liver cancer and significiance in conventional treatment. Shijie Huaren Xiaohua Zazhi. 2001;9:1449-1451. |
| 3. | Lau WY. Management of hepatocellular carcinoma. J R Coll Surg Edinb. 2002;47:389-399. [PubMed] |
| 4. | Achenbach T, Seifert JK, Pitton MB, Schunk K, Junginger T. Chemoembolization for primary liver cancer. Eur J Surg Oncol. 2002;28:37-41. [PubMed] [DOI] |
| 5. | Alsowmely AM, Hodgson HJ. Non-surgical treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2002;16:1-15. [PubMed] [DOI] |
| 6. | Lin Z, Ren Z, Xia J. Appraisal of postoperative transcatheter arterial chemoembolization(TACE) for prevention and treatment of hepatocellular carcinoma recurrence. Zhonghua Zhongliu Zazhi. 2000;22:315-317. [PubMed] |
| 7. | Sakon M, Nagano H, Nakamori S, Dono K, Umeshita K, Murakami T, Nakamura H, Monden M. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg. 2002;137:94-99. [PubMed] [DOI] |
| 8. | Liu L, Huang PL, Tong GS. Experimental study of the antitumor effect of phosphorus-32 glass microspheres on the tumor loaded nude mice. World J Gastroenterol. 2000;6:58. |
| 9. | Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211-221. [DOI] |
| 10. | Maeda S, Fujiyama S, Tanaka M, Ashihara H, Hirata R, Tomita K. Survival and local recurrence rates of hepatocellular carcinoma patients treated by transarterial chemolipiodolization with and without embolization. Hepatol Res. 2002;23:202-210. [DOI] |
| 11. | Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747-1752. [PubMed] [DOI] |
| 12. | Georgiades CS, Ramsey DE, Solomon S, Geschwind JF. New nonsurgical therapies in the treatment of hepatocellular carcinoma. Tech Vasc Interv Radiol. 2001;4:193-199. [DOI] |
| 13. | Kyotani S, Nishioka Y. The development of embolizing materials for chemo-embolization therapy of hepatocellular carcinoma. Yakugaku Zasshi. 2000;120:1173-1184. [PubMed] [DOI] |
| 14. | Takeuchi I, Ishida H, Inokuma S, Nakada H, Oosawa T, Sakimoto T, Hashimoto D, Kashimada A, Osada H. Hepatic arterial injection of degradable starch microspheres (DSM) combined with adriamycin (ADM) and mitomycin C (MMC) in patients with liver metastasis of colorectal cancer. Gan To Kagaku Ryoho. 2000;27:1904-1906. [PubMed] |
| 15. | Kumada T, Nakano S, Sone Y, Kiriyama S, Hisanaga Y, Rikitoku T, Tamoto A, Honda T. Clinical effectiveness of degradable starch microspheres (DSM) in patients with liver cancer. Gan To Kagaku Ryoho. 1999;26:1678-1683. [PubMed] |
| 16. | Qu W, Chen QH, Zhao RQ, Li N, Zhou MH. Study on biodegradable sustained release alarelin microspheres injection. Zhonguo Yiyao Gongye Zazhi. 2000;31:14-18. |
| 17. | Trubenbach J, Graepler F, Pereira PL, Ruck P, Lauer U, Gregor-M , Claussen CD, Huppert PE. Growth characteristics and imaging properties of the morris hepatoma 3 924 A in ACI rats: a suitable model for transarterial chemoembolization. Cardiovasc Intervent Radiol. 2000;23:211-217. [PubMed] [DOI] |
| 18. | Zhang JS, Wang H, Huang WQ, Sun L, Huang GS, Zhang YQ. Grouth inhibition of luteinzing hormone-releasing hormone analog on hHCC hepatocarcinoma cell xenografts in nude mice. Shijie Huaren Xiaohua Zazhi. 2002;10:759-764. |
| 19. | Minamimura T, Sato H, Kasaoka S, Saito T, Ishizawa S, Takemori S, Tazawa K, Tsukada K. Tumor regression by inductive hyperthermia combined with hepatic embolization using dextran magnetite-incorporated microspheres in rats. AInt J Oncol. 2000;16:1153-1158. [DOI] |
| 20. | Cao W, Wang ZM, Liang ZH, Zhang HX, Wang YQ, Guan Y, Li WX, Pan BR. Effects of angiogenesis inhibitor TNP-470 with lipiodol in arterial embolization of liver cancer in rabbit model. Shijie Huaren Xiaohua Zazhi. 2000;8:629-632. |
| 21. | Wu W, Deng R, Ou Y. Therapeutic efficacy of microsphere-entrapped curcuma aromatica oil infused via hepatic artery against transplanted hepatoma in rats. Zhonghua Ganzangbing Zazhi. 2000;8:24-26. [PubMed] |
| 22. | Lu CD, Peng SY, Jiang XC, Chiba Y, Tanigawa N. Preoperative transcatheter arterial chemoembolization and prognosis of patients with hepatocellular carcinomas: retrospective analysis of 120 cases. World J Surg. 1999;23:293-300. [DOI] |
| 23. | Huang YS, Chiang JH, Wu JC, Chang FY, Lee SD. Risk of hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma: predictive value of the monoethylglycin- exylidide test. Am J Gastroenterol. 2002;97:1223-1227. [PubMed] [DOI] |
| 24. | Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellularcarcinoma. Cancer. 2002;94:1747-1752. [PubMed] [DOI] |
| 25. | Kim YB, Park YN, Park C. Increased proliferation activities of vascular endothelial cells and tumour cells in residual hepatocellular carcinoma following transcatheter arterial embolization. Histopathology. 2001;38:160-166. [DOI] |
| 26. | Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, Yoon HK, Sung KB, Lee YS, Suh DJ. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002;17:52-58. [DOI] |
| 27. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] [DOI] |
| 28. | Loewe C, Cejna M, Schoder M, Thurnher MM, Lammer J, Thurnher SA. Arterial embolization of unresectable hepatocellular carcinoma with use of cyanoacrylate and lipiodol. J Vasc Interv Radiol. 2002;13:61-69. [DOI] |
| 29. | Pohlen U, Berger G, Binnenhei M, Reszka R, Buhr HJ. Increased carboplatin concentration in liver tumors through temporary flow retardation with starch microspheres (Spherex) and gelatin powder (Gelfoam): an experimental study in liver tumor-bearing rabbits. J Surg Res. 2000;92:165-170. [PubMed] [DOI] |
| 30. | Tamura T, Fujita F, Tanimoto M, Koike M, Suzuki A, Fujita M, Horikiri Y, Sakamoto Y, Suzuki T, Yoshino H. Anti-tumor effect of intraperitoneal administration of cisplatin-loaded microspheres to human tumor xenografted nude mice. J Control Release. 2002;80:295-307. [DOI] |
| 31. | Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Fujita M, Hasegawa T, Kitabatake S, Miyazaki K, Ishiguro Y. A new embolic agent--DSM(degradable starch microsphere. Nippon Rinsho. 2001;59:539-544. [PubMed] |
| 32. | Nijsen F, Rook D, Brandt C, Meijer R, Dullens H, Zonnenberg B, de Klerk J, van Rijk P, Hennink W, van het Schip F. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: a biodistribution study. Eur J Nucl Med. 2001;28:743-749. [DOI] |
| 33. | Kubo H, Loegering DA, Adolphson CR, Gleich GJ. Cytotoxic properties of eosinophil granule major basic protein for tumor cells. Int Arch Allergy Immunol. 1999;118:426-428. [PubMed] [DOI] |
| 34. | Lee SY, Park HS, Lee SJ, Choi MU. Melittin exerts multiple effects on the release of free fatty acids from L1210 cells: lack of selective activation of phospholipase A2 by melittin. Arch Biochem Biophys. 2001;389:57-67. [PubMed] [DOI] |