修回日期: 2002-06-20
接受日期: 2002-07-12
在线出版日期: 2003-05-15
观察表皮生长因子(EGF)对大鼠肠缺血-再灌注(I/R)后磷酸化细胞外信号调节激酶(phospho-p44/42 MAPK)表达的影响.
夹闭大鼠肠系膜上动脉根部45 min之后放松血管夹形成再灌注, 同时经颈静脉分别注入EGF 100 μg/kg或生理盐水, 分别于伤后2、6、12和24 h将动物活杀. 设置对照组和单纯缺血组. 检测血浆D-乳酸浓度, 取小肠组织进行形态学观察, 用免疫组织化学方法研究磷酸化p44/42 MAPK的表达.
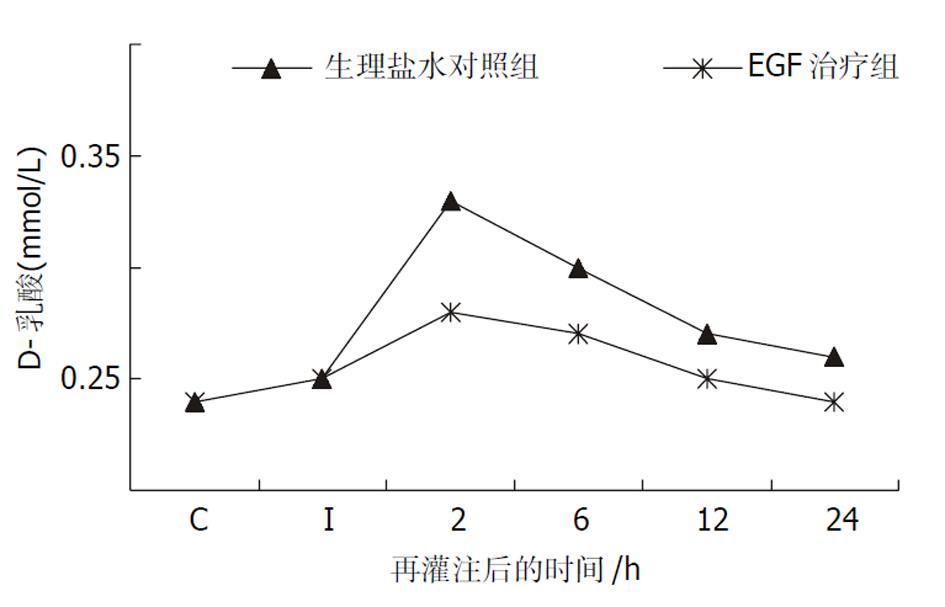
(1)再灌注后血浆D-乳酸水平升高, 小肠黏膜充血、水肿、炎细胞浸润及坏死糜烂, 再灌注后6 h最显著.EGF显著降低D-乳酸水平升高的幅度(P<0.05), 明显改善I/R引起的病理损害. (2)磷酸化p44/42 MAPK染色显示, 正常大鼠的绒毛上皮、陷窝和固有层细胞均有阳性颗粒, 主要存在于胞质内. 缺血和再灌注后阳性细胞数量显著增加, 随再灌注时间的延长, 阳性颗粒逐渐转位入核, 在12 h最显著. 24 h主要表现为胞质内表达. EGF治疗后阳性细胞数量增多, 细胞核表达的数量增加.
肠道I/R损伤激活磷酸化p44/42 MAPK的表达, EGF促进p44/42 MAPK的早期表达与核转位, 从而参与细胞的应激反应和增生与分化.
引文著录: 李平, 邢峰, 付小兵, 杨银辉, 郭宝琛. EGF对小肠缺血再灌注后磷酸化p44/42 MAPK表达的影响. 世界华人消化杂志 2003; 11(5): 578-582
Revised: June 20, 2002
Accepted: July 12, 2002
Published online: May 15, 2003
To investigate the effects of EGF on the characteristics of phosphrylated p44/42 MAPK expression and its biological significance in EGF-induced gut repair after ischemia-reperfusion (I/R) injury.
A total of 80 Wistar rats were randomly divided into four groups, namely EGF treated group (E), normal saline control (R), ischemia group (I) and sham operated control (C). In group E and R, the rats were treated with intravenous EGF 100 μg/kg/rat or normal saline respectively after 45 minutes of superior mesenteric artery occlusion. Blood samples were collected at 2, 6, 12 and 24 hours after reperfusion and plasma D-lactate were determined. Tissue samples from intestine were also taken for histological analysis and immunohistochemical analysis of phospho-p44/42 MAPK.
The changes of histological structure and D-lactate indicated that the intestinal barrier was damaged after intestinal I/R injury, while EGF treatment significantly improved the outcome. In group C and I positive signals of phospho-p44/42 MAPK were mainly located in the cytoplasm of the intestinal villi and crypts, while in group I positive cells increased significantly (P<0.05). In group R, positive signals were found in almost all the cells and the percentage of positive nuclei increased with the time of reperfusion, reaching its peak after 12h of reperfusion. In group E, the percentages were higher than those in group R and the peak of nuclear expression was earlier.
EGF administration improves the outcome of I/R induced intestinal damage. After I/R the expression and nuclear translocation of phspho-p44/42 MAPK increases with the time of reperfusion, suggesting its role in intestinal reconstitution. EGF treatment induces its early expression and translocation into the nucleus, suggesting the significance of p44/42 MAPK signaling pathway in EGF-induced gut repair.
- Citation: Li P, Xin F, Fu XB, Yang YH, Guo BC. Effects of EGF on expression of phosphorylated p44/42 MAPK in rat small intestine after ischemia-reperfusion injury. Shijie Huaren Xiaohua Zazhi 2003; 11(5): 578-582
- URL: https://www.wjgnet.com/1009-3079/full/v11/i5/578.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i5.578
利用生长因子调控损伤修复的意义已经引起人们的重视[1], 表皮生长因子(EGF)参与内脏损伤后的主动修复过程也已得到实验证实[2-5]. 与此同时, 细胞外信号调节激酶(extracellular-signal regulated protein kinase, p44/42 MAPK)在EGF等生长因子刺激引起的细胞反应中起重要调控作用[6-8]. 体外研究证实, EGF可以通过激活p44/42 MAPK通路促进小肠细胞的增生反应[9-11]. 本实验利用大鼠肠I/R损伤模型, 给予大鼠静脉注射EGF, 观察血浆D-乳酸浓度的变化规律以及相应的病理学改变, 用免疫组织化学方法研究肠I/R引起的p44/42 MAPK的表达规律以及EGF对p44/42 MAPK表达的影响, 进而探讨其在创伤修复中的作用.
♂Wistar大鼠, 体质量200-250 g(购自军事医学科学院动物中心), 随机分为对照组(C)、肠缺血组(I)、肠缺血-再灌注组(R)和EGF治疗组(E). 根据缺血后再灌注时间的不同将R组和E组又分成2, 6, 12和24 h共4组(R2, R6, R12, R24以及E2, E6, E12, E24), 每组8只动物. 实验前禁食12 h, 自由饮水. 采用肠系膜上动脉(SMA)夹闭-松夹方式制成肠缺血-再灌注模型. 大鼠腹腔注射30 g/L戊巴比妥钠(35 mg/kg)麻醉, 常规消毒后取腹正中切口3-4 cm, 钝性分离SMA根部. E组和R组动物以血管夹夹闭SMA根部, 完全阻断血流45 min之后放松血管夹, 使肠道血流恢复形成再灌注, 在松开动脉夹的同时经右颈静脉注入EGF 100 μg/kg(由中国科学院上海生物化学研究所提供)或生理盐水0.5 mL. 假手术组仅分离SMA不作夹闭, I组不注射EGF或生理盐水.各组动物于关闭腹腔后皮下注射10 ml生理盐水抗休克.
按照设定时间点将动物处死, 取动物肠道石蜡包埋后切片、HE染色, 光镜下观察. 取门静脉血离心后分离血浆, -80 ℃保存, 用酶联紫外分光光度法测定血浆D-乳酸水平. 动物肠道经40 g/L甲醛固定、脱水、石蜡包埋、切片后, 应用PowerVisionTM二步法研究磷酸化p44/42 MAPK的表达. 采用磷酸化p44/42 MAPK小鼠单克隆抗体(cell signaling technology, Inc, 美国)以及相应的第二抗体(北京中山生物技术有限公司), DAB(二氨基联苯胺, 福州迈新生物技术公司), 按照试剂说明书要求进行免疫组织化学技术操作. 石蜡切片按常规脱蜡至水, 在体积分数为30 ml/L H2O2中孵育10 min, 以消除内源性过氧化物酶活性. PBS冲洗, 置于0.01 mol/L, pH6.0的枸橼酸盐缓冲液中行抗原热修复后, 滴加按1:100稀释的一抗, 37 ℃孵育40 min. PBS冲洗, 滴加辣根过氧化物酶(HRP)标记的二抗, 37 ℃孵育20 min, PBS漂洗后DAB显色, 苏木素复染, 常规脱水, 透明, 封片, 显微镜下观察, 结果以细胞质和/或细胞核着棕色者为阳性染色. 另用PBS代替一抗做阴性对照. 光镜下观察阳性细胞在小肠黏膜的分布. 每只大鼠观察50个纵向切开的陷窝和绒毛(陷窝腔和绒毛应保持完整), 统计阳性细胞百分率, 其结果以均数±标准差(x±s)表示, SAS软件包统计各组间差异.
大鼠SMA夹闭后, 肠道明显变紫, 蠕动减慢. HE染色见黏膜上皮出现充血、水肿和炎细胞浸润以及糜烂、坏死, 以伤后6 h最明显, 在再灌注后24 h基本恢复正常的黏膜结构, E组动物肠黏膜损伤的程度较R组明显减轻.
D-乳酸是肠道细菌特有的代谢产物, 血浆中D-乳酸含量的变化可反映肠黏膜通透性的改变[12,13]. 肠缺血45 min后, D-乳酸浓度尚未发生明显改变. 再灌注2 h和6 h, 各组动物血浆D-乳酸浓度较假手术组均显著升高, 但R组升高的幅度显著高于E组(P<0.01或P<0.05, 图1).
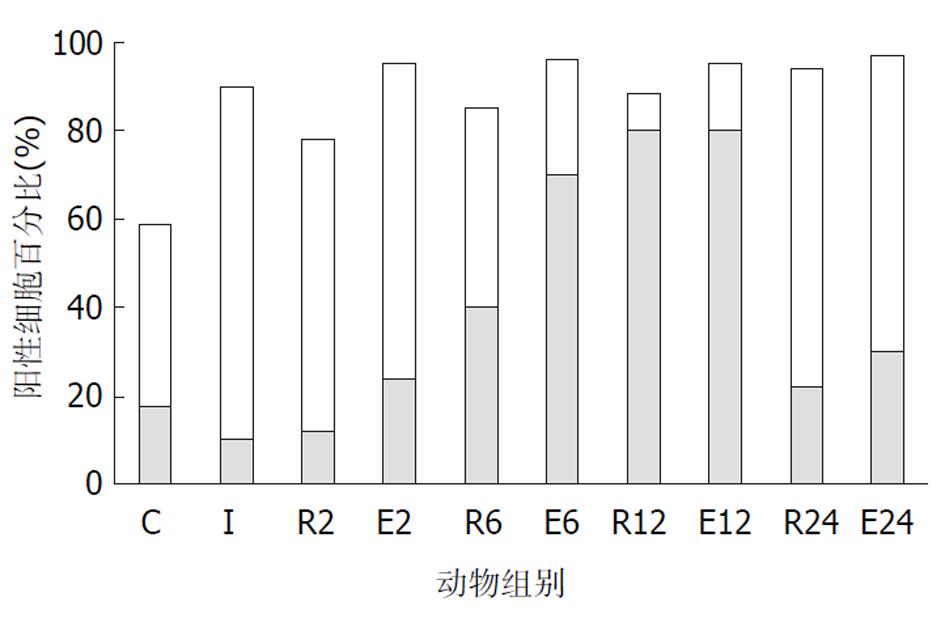
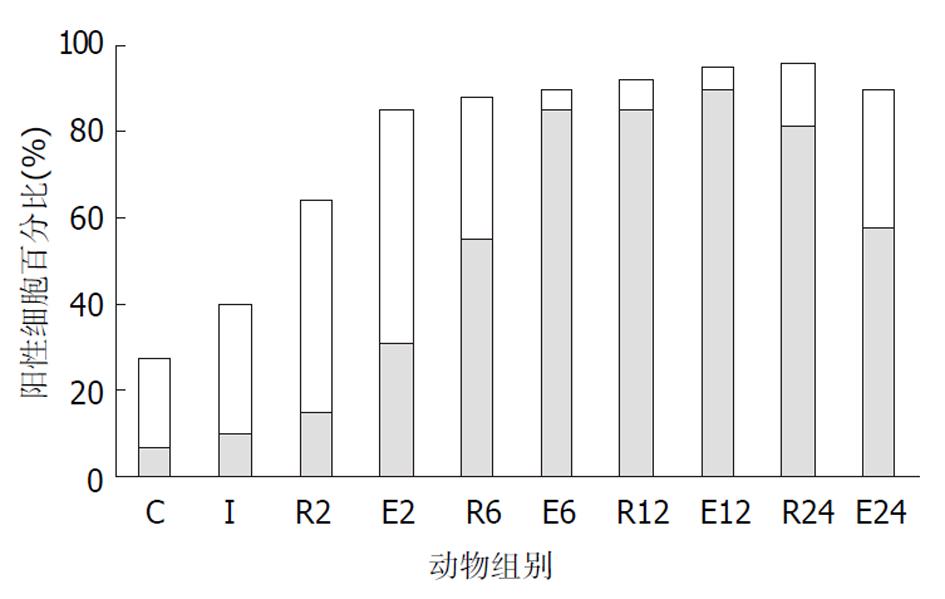
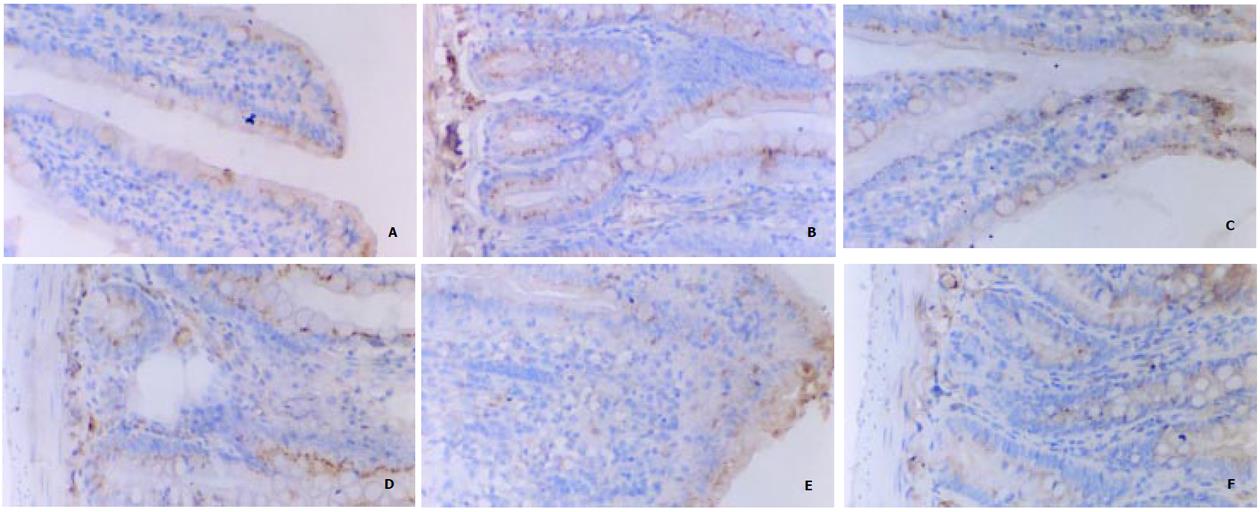
C组、I组和R组: 在正常和伤后大鼠, 绒毛上皮、小肠陷窝和固有层均可见到染色阳性的细胞.在C组大鼠的绒毛上皮和小肠陷窝, 阳性细胞的比例分别为59%和27.5%, 表现为散在分布于胞质内的棕色颗粒, 大部分靠近细胞核, 少量细胞有核内表达.小肠陷窝核内表达p44/42 MAPK的细胞主要位于陷窝的中下部, 相当于干细胞、短暂扩增细胞及其初级子代细胞的位置. 杯状细胞的胞质内也可见阳性颗粒. I组大鼠90%绒毛上皮细胞和40%陷窝细胞的胞质内出现阳性颗粒, 核内表达无明显改变. R组几乎全部绒毛上皮细胞和陷窝细胞表达磷酸化p44/42 MAPK, 但随着再灌注时间的延长, 核内表达的比例增加. 在R2组和R6组阳性颗粒主要位于胞质中, R12组则主要位于细胞核, R24组核内表达的比例下降接近C组. 各组动物的黏膜固有层均有少量的阳性细胞, 正常情况下主要位于胞质, 随再灌注时间延长核内表达增多, 在R12组最显著(图2, 图3, 图4). E组绒毛上皮细胞和陷窝的阳性细胞数量均高于R组相应时相点, 核内表达的比例也高于R组. E6和E12组主要表现为核内表达, 以E6最为显著. E2和E24组主要为胞质内表达(图2-4).
EGF是强有力的促细胞分裂因子[14,15], 对胃肠道黏膜细胞具有促进生长和增生的作用[16-21], p44/42 MAPK在胞质内广泛分布, 在未受刺激的细胞内, 主要表现为脱磷酸型, 其苏氨酸和酪氨酸残基被磷酸化后发生激活, 主要与细胞的增生通路有关[22,23], 已证明p44/42 MAPK途径是EGF诱导表皮成纤维细胞、角质细胞、小肠癌细胞IEC-6等细胞增生的主要细胞内信号传导通路[24-27]. 在心、肾、肺等多种组织中, 均发现I/R损伤可迅速激活MAPK信号传导通路, 作为早期细胞内信号参与细胞对应激反应的调节. 一般认为I/R时p44/42 MAPK活性的增加是细胞针对缺氧刺激启动修复过程、促进细胞存活的保护性机制, 对于经历了I/R损伤的细胞具有保护作用. 在MAPK信号途径中的各个组成成分如p44/42 MAPK、c-fos的激活均具有相似的分子机制, 都需要保守位点上的双磷酸化作用, 信号转导最终通过基因表达来实现细胞调控, p44/42 MAPK的磷酸化是p44/42 MAPK正在发生功能活动的标志[28]. EGF与其受体(EGFR)结合后, 再与ras结合, 进而激活raf-1, raf-1接着激活MEK1/MEK2(p44/42 MAPK的上游激酶), 进而激活p44/42 MAPK, 后者进入核内, 通过中间反应物介导原癌基因c-myc、c-fos、c-jun、Elk1、TAL1等的产生和磷酸化, 影响下游基因的表达, 最终导致细胞的增生反应[29-33]. p44/42 MAPK被激活后也可以停留在胞质中, 作用于细胞表面分子如EGFR、磷脂酶A2, 启动多条细胞内信号传导通路, 介导胞外环境应激条件信号, 引起细胞内的抗应激反应.
p44/42 MAPK被激活后, 可以表现为持久激活或短暂激活, 持久激活(活性高)的p44/42 MAPK可部分转入核内, 可以使相应的转录因子发生磷酸化, 而短暂激活(活性低)的p44/42 MAPK不能进入核内, 二者由于"入核量"的差异使细胞表达不同质或量的产物, 从而产生不同的细胞生物学效应, 因此p44/42 MAPK在转录水平上的差异可以使其产生不同的效应.
本实验中, 在假手术组大鼠的小肠黏膜上皮细胞内, 磷酸化p44/42 MAPK主要存在于胞质, 核内表达较少. 缺血45 min后, 单层柱状上皮和小肠陷窝阳性细胞的比例显著增加, 表明缺血可以迅速激活p44/42 MAPK通路, 此时仍主要为胞质内表达. 随着再灌注时间的延长, 几乎全部细胞表达磷酸化p44/42 MAPK, 而肠道组织学改变和血浆D-乳酸水平的变化表明I/R引起肠道损伤, 提示I/R损伤持续激活p44/42 MAPK通路, 使其参与了I/R引起的应激反应. 值得注意的是, 无论在绒毛还是陷窝, 核内表达磷酸化p44/42 MAPK的细胞数量均随再灌注时间延长而增加, 而进入核内是p44/42 MAPK发挥促进细胞增生作用的前提. R组大鼠核内表达p44/42 MAPK的高峰在再灌注后12 h, EGF治疗组核内表达的时间较R组提前, 持续时间也较R组延长.由此看来, EGF促进了p44/42 MAPK的早期激活和核转位.由于绒毛单层柱状上皮主要为分化细胞, 陷窝主要由干细胞、短暂扩充细胞和较初级的增生细胞组成, 磷酸化p44/42 MAPK在这些部位的活跃表达表明他与肠道细胞(包括干细胞)的增生与分化有密切联系. 本结果表明, 外源性EGF参与了肠道内脏损伤的修复过程, 其作用是通过早期激活p44/42 MAPK通路, 参与细胞的应激反应, 促进细胞的增生与分化. 该结果进一步证实了我们以前所观察到的采用外源性补充生长因子对缺血性内脏治疗作用的理论[34].
| 2. | Leeb SN, Vogl D, Falk W, Scholmerich J, Rogler G, Gelbmann CM. Regulation of migration of human colonic myofibroblasts. Growth Factors. 2002;20:81-91. [PubMed] [DOI] |
| 3. | Rongione AJ, Kusske AM, Newton TR, Ashley SW, Zinner MJ, Mcfadden DW. EGF and TGF stimulate proabsorption of glucose and electrolytes by Na+/glucose cotransporter in awake canine model. Dig Dis Sci. 2001;46:1740-1747. [DOI] |
| 4. | Dignass AU, Sturm A. Peptide growth factors in the intestine. Eur J Gastroenterol Hepatol. 2001;13:763-770. [PubMed] [DOI] |
| 5. | Eizaguirre I, Aldazabal P, Barrena MJ, Garcia-Arenzana JM, Ariz C, Candelas S, Tovar JA. Effect of growth hormone, epidermal growth factor, and insulin on bacterial translocation in experimental short bowel syndrome. J Pediatr Surg. 2000;35:692-695. [PubMed] [DOI] |
| 6. | Koul S, Chaturvedi LS, Sekhon A, Bhandari A, Menon M, Koul HK. Effects of oxalate on the re-initiation of DNA synthesis in LLC-PK1 cells do not involve p42/44 MAP kinase activation. Kidney Int. 2002;61:525-533. [PubMed] [DOI] |
| 7. | Llorens F, Garcia L, Itarte E, Gomez N. Apigenin and LY294002 prolong EGF-stimulated ERK1/2 activation in PC12 cells but are unable to induce full differentiation. FEBS Lett. 2002;510:149-153. [DOI] |
| 8. | Sah JF, Eckert RL, Chandraratna RA, Rorke EA. Retinoids suppress epidermal growth factor-associated cell proliferation by inhibiting epidermal growth factor receptor-dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735. [PubMed] [DOI] |
| 9. | Tanimura S, Nomura K, Ozaki K, Tsujimoto M, Kondo T, Kohno M. Prolonged nuclear retention of activated extracellular signal-regulated kinase 1/2 is required for hepatocyte growth factor-induced cell motility. J Biol Chem. 2002;277:28256-28264. [PubMed] [DOI] |
| 10. | Lewis MD, Ham J, Rees DA, Lewis BM, Scanlon MF. Mitogen-activated protein kinase mediates epidermal growth factor-induced morphogenesis in pituitary GH3 cells. J Neuroendocrinol. 2002;14:361-367. [DOI] |
| 11. | Marques SA, Dy LC, Southall MD, Yi Q, Smietana E, Kapur R, Marques M, Travers JB, Spandau DF. The platelet-activating factor receptor activates the extracellular signal-regulated kinase mitogen-activated protein kinase and induces proliferation of epidermal cells through an epidermal growth factor-receptor-dependent pathway. J Pharmacol Exp Ther. 2002;300:1026-1035. [DOI] |
| 13. | Sun XQ, Fu XB, Zhang R, L Y, Deng Q, Jiang XG, Sheng ZY. Relationship between plasma D(-)lactate and intestinal damage after severe injuries in rats. World J Gastroenterol. 2001;7:555-558. [DOI] |
| 15. | Xia L, Yuan YZ, Xu CD, Zhang YP, Qiao MM, Xu JX. Effects of epidermal growth factor on the growth of human gastric cancer cell and the implanted tumor of nude mice. World J Gastroenterol. 2002;8:455-458. [PubMed] [DOI] |
| 16. | Chen DL, Wang WZ, Wang JY. Epidermal growth factor prevents gut atro phy and maintains intestinal integrity in rats with acute pancreatitis. World J Gastroentero. 2000;6:762-765. [DOI] |
| 17. | Berlanga-Acosta J, Playford RJ, Mandir N. Gastrointestinal cell proliferation and crypt fission are separate but complementary means of increasing tissue mass following infusion of epidermal growth factor in rats. Gut. 2001;48:803-807. [DOI] |
| 18. | Reindel JF, Gough AW, Pilcher GD, Bobrowski WF, Sobocinski GP, de la Iglesia FA. Systemic proliferative changes and clinical signs in cynomolgus monkeys administered a recombinant derivative of human epidermal growth factor. Toxicol Pathol. 2001;29:159-173. [PubMed] [DOI] |
| 19. | Avissar NE, Ziegler TR, Wang HT, Gu LH, Miller JH, Iannoli P, Leibach FH, Ganapathy V, Sax HC. Growth factors regulation of rabbit sodium-dependent neutral amino acid transporter ATB0 and oligopeptide transporter 1 mRNAs expression after enteretomy. J Parenter Enteral Nutr. 2001;25:65-72. [PubMed] [DOI] |
| 20. | Duh G, Mouri N, Warburton D, Thomas DW. EGF regulates early embryonic mouse gut development in chemically defined organ culture. Pediatr Res. 2000;48:794-802. [PubMed] [DOI] |
| 21. | Stern LE, Erwin CR, O'Brien DP, Huang F, Warner BW. Epidermal growth factor is critical for intestinal adaptation following small bowel resection. Microsc Res Tech. 2000;51:138-148. [DOI] |
| 22. | Zhao D, Letterman J, Schreiber BM. Beta-Migrating very low density lipoprotein (beta VLDL) activates smooth muscle cell mitogen-activated protein (MAP) kinase via G protein-coupled receptor-mediated transactivation of the epidermal growth factor (EGF) receptor: effect of MAP kinase activation on beta VLDL plus EGF-induced cell proliferation. J Biol Chem. 2001;276:30579-30588. [PubMed] [DOI] |
| 23. | Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2001;24:405-413. [PubMed] [DOI] |
| 24. | Kawahara E, Nakada N, Hikichi T, Kobayashi J, Nakanishi I. EGF and beta1 integrin convergently regulate migration of A431 carcinoma cell through MAP kinase activation. Exp Cell Res. 2002;272:84-91. [PubMed] [DOI] |
| 25. | Cras-Meneur C, Elghazi L, Czernichow P, Scharfmann R. Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation. Diabetes. 2001;50:1571-1579. [DOI] |
| 26. | Thrane EV, Schwarze PE, Thoresen GH, Lag M, Refsnes M. Persistent versus transient map kinase (ERK) activation in the proliferation of lung epithelial type 2 cells. Exp Lung Res. 2001;27:387-400. [DOI] |
| 27. | Harris VK, Kagan BL, Ray R, Coticchia CM, Liaudet-Coopman ED, Wellstein A, Tate Riegel A. Serum induction of the fibroblast growth factor-binding protein (FGF-BP) is mediated through ERK and p38 MAP kinase activation and C/EBP-regulated transcription. Oncogene. 2001;20:1730-1738. [PubMed] [DOI] |
| 28. | Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33-36. [DOI] |
| 29. | Lee JW, Juliano RL. The alpha5beta1 integrin selectively enhances epidermal growth factor signaling to the phosphatidylinositol-3-kinase/Akt pathway in intestinal epithelial cells. Biochim Biophys Acta. 2002;1542:23-31. [DOI] |
| 30. | Oksvold MP, Skarpen E, Wierod L, Paulsen RE, Huitfeldt HS. Re-localization of activated EGF receptor and its signal transducers to multivesicular compartments downstream of early endosomes in response to EGF. Eur J Cell Biol. 2001;80:285-294. [PubMed] [DOI] |
| 31. | Jost M, Huggett TM, Kari C, Rodeck U. Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol Biol Cell. 2001;12:1519-1527. [PubMed] [DOI] |
| 32. | Pierce KL, Tohgo A, Ahn S, Field ME, Luttrell LM, Lefkowitz RJ. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J Biol Chem. 2001;276:23155-231160. [PubMed] [DOI] |
| 34. | Fu XB, Yang YH, Sun TZ, Gu XM, Jiang LX, Sun XQ, Sheng ZY. Effect of intestinal ischemia-reperfusion on expressions of endogenous basic fibroblast growth factor and transforming growth factor ? in lung and its relation with lung repair. World J Gastroenterol. 2000;6:353-355. [DOI] |