INTRODUCTION
β2GPI (beta-2-glycoproteinI) is a plasm glycoprotein circulating as a free protein and is associated to lipoproteins. This protein is also referred to as apolipoprotein H(Apo H). Human β2GPI is a single-chain molecule consisting of 326 amino acid residues and 5 carbohydrate chain and has a molecule mass of approximately 55 kDa[1-3]. The amino acid sequences of β2GPI in human, bovine, mouse, and rat appear to be highly conserved[4-6]. The protein contains 5 internal repeat unit of 60 amino acid residues, each with 2 internal disulfide bonds, known as Sushi domain[7].
Although quite a lot is known about the structure of β2GPI, its biological function remains unclear. It is known that β2GPI can bind to negatively charged substances such as DNA and heparin and negatively charged phospholipids etc in vivo[8,9], but the meaning of such interaction is still unclear. It is known that β2GPI may serve as a major factor in clearing the plasma liposome[10] as well as an anticoagulant in blood[11]. β2GPI may also modulate the function of kidney and placenta. The abnormality of plasma β2GPI level has been shown to be associated with many diseases such as arterial and venous thrombosis, recurrent abortion and alcoholic liver disease[12,13]. In addition, it has been found that, in diabetes or atherosclerosis patients, the concentration of plasma β2GPI increases and distribution of the protein among different types of lipoprotein is perturbed[14,15]. Recent research on β2GPI has given a further impetus to the discovery that lipid-associated β2GPI can bind to some pathogenic antigens or proteins such as HBV Dane particles, protein p18, p26, gp160 of HIV and so on[16], and perhaps hepatitis virus antigen[17,18]. These findings highlighted a potentially critical role of β2GPI in the mechanism of hepatitis B, AIDS, and systemic lupus erythematosus, etc.
The lipid-binding and transportation functions are considered as a basic mechanism related to its physiological and pathogenic functions. It has been demonstrated by several labs that β2GPI prefers to bind negatively charged phospholipids[9,19-21]. It was reported that β2GPI could be removed from the membranes with a weakly acidic buffer and it was partly associated with chylomicrons and high-density lipoproteins, both of which were targeted to hepatocytes during the normal course of lipid metabolism[22]. In the present paper, the characteristics of β2GPI interacting with HBsAg were further examined by several measurements. As a result, the standpoints that β2GPI might participate in HBV infection were held out accordingly. Our results may also help to explain how β2GPI facilitates HBV transportation, location and the important roles of β2GPI in HBV infection.
MATERIALS AND METHODS
Reagents
TEMED, APS, PMSF, and aprotinin purchased from Sigma Chemical Co., acrylamide and biacrylamide purchased from Serva, human β2GPI provided by Prof. Zhang GR from Jilin University, rHBsAg offered by Zhang HY from Jilin Institute of Family Plan, Hybond obtained from Amersham, SDS, NP-40, Coomasie brilliant blue obtained from Fluka (Finland). The other chemicals used were of analytical grade made in China.
ELISA-based determination of β2GPI-rHBsAg interaction
rHBsAg was diluted into 0.125 μg/mL, 0.5 μg/mL and 2 μg/mL in 0.05 NaHCO3 (pH9.4) and added to 96-well plates respectively at 4 °C overnight (8 wells/group). Non-specific sites were blocked with PBS containing 1% BSA for 1.5 h at 37 °C. Plates were washed three times between the different incubation steps. Biotinylated β2GPI was added to every well followed by adding HRP-Avidin and substrate consequentially. Simultaneously, 40 ng of non-labeled β2GPI was added to 4well/group as a competitor. OPD was used to develop color and the intensity of color was quantified at 492 nm with Model 550 Microplate Reader (Bio-Rad). In addition, BSA was used as negative control (2 μg/mL, 1 μg/mL, 0.2 μg/mL), to observe its binding to β2GPI.
SDS-PAGE and ligand blotting
The β2GPI protein was performed by SDS-PAGE using the vertical electrophoritic apparatus (Bio-Rad) on 12% acrylamide under reduced and non-reduced condition. The gels were stained with Coomassie brilliant blue to visualize the proteins. The proteins separated were transferred onto nitrocellulose using an electric transfer system at 50 V for 16 min, followed by immunoblot analysis employing biotinylated rHBsAg as a detecting probe and DAB as a developing system. The blotting buffer consisted of phosphate-buffered saline (10 mM phosphate, pH7.5, 138 mM NaCl, 2.7 mM KCl) containing 5% dried skimmed milk powder and 1% Tween 20 detergent (Sigma). Biotinylated rHBsAg was used at a dilution of 1/200 and HRP-avidin (Huamei Company), a dilution of 1/200. The density was analyzed with Luzex-F Image-analysis System.
Statistical analysis
Results from quantitative parameters were presented as mean ± SD and comparisons of which were performed using the Student’s t test.
RESULTS
ELISA-based determination of β2GPI-rHBsAg interaction
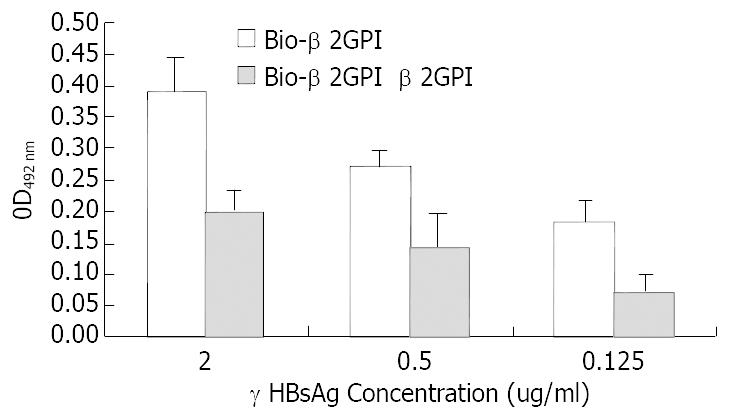
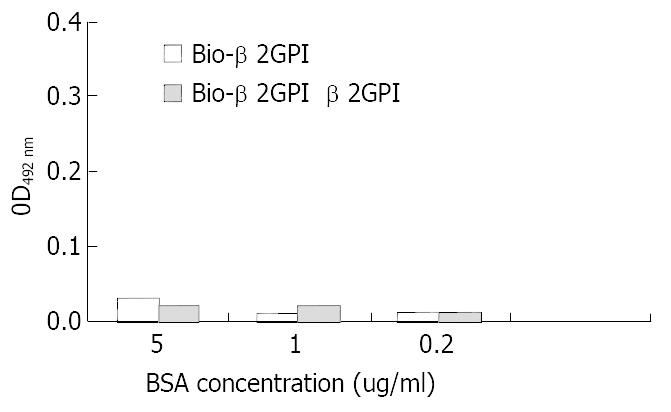
rHBsAg was diluted into 0.125 μg/mL, 0.5 μg/mL and 2 μg/mL and used as capture antibody to coat the plates, then biotinlyated β2GPI was added to interact with rHBsAg, simultaneously we used non-biotinlyated β2GPI to observe the competitive binding interaction. The following figure indicated that with the increase of rHBsAg the binding of β2GPIto rHBsAg elevated and these changes had statistic significance (vs 0.125 μg/mL group, P < 0.05). When we added non-biotinlyated β2GPI, the OD value significantly decreased though they still were positively relevant to rHBsAg, suggesting that non-biotinlyated β2GPI competed with biotinlyated β2GPI to saturate the binding sites on rHBsAg (Figure 1). Meanwhile BSA was used as negative control to substitute for rHBsAg coating the plates, the results indicated no interaction between β2GPI and BSA, (Figure 2) suggesting the affinity of β2GPI to rHBsAg was specific.
Figure 1 Comparative binding reaction of β2GPI to rHBsAg by ELISA-based determination of protein interaction.
Figure 2 Comparative binding reaction of β2GPI to BSA by ELISA-based determination of protein interaction.
Binding of β2GPI to rHBsAg observed by ligand blotting
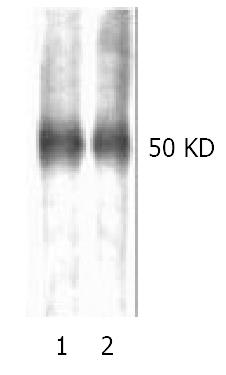
The discrepancy between ligand blotting and Western blotting was that the former made use of ligand-receptor reaction, while the latter, the antigen-antibody interaction. The image showing -50 kDa protein bands in both lane 1 and lane 2 represented reduced (with DTT) and non-reduced (without DTT) condition, respectively (Figure 3). The results indicated that β2GPI might bind to rHBsAg no matter whether it was under reduced condition or not, which manifested difference from that reported by Mehdi et al[17]. The subsequent digital scaning by Luzex-F Imager confirmed no quantitative changes in density between lane1 and lane 2.
Figure 3 Reduced (lane1) and non-reduced (lane2) human β2GPI (1 μg/lane) was separated with 12% SDS-PAGE and applied to Hybond nitrocellous membrane, blocked with 5% skimmed milk, then probed with biotinylated rHBsAg, color was developed with HRP-avidin in DAB solution.
Single band at -50 kDa was observed, no significant diference in color den-sity was observd, which was further confirmed by digital scaning.
DISCUSSION
Hepatitis B virus (HBV) is a member of the hepadnavirus family, which includes duck HBV, woodchuck hepatitis virus and ground squirrel hepatitis virus. These viruses are highly infectious for their host animals, targeting primarily, though not exclusively, the liver. Although many researches have been done, it is not clear which of these virus’ components is in responsible for its attachment to a target cell[23]. As a step toward identifying the mechanism of HBV targeting hepatocytes, Haider Mehdi reported that a 50 kDa protein known as β2GPI, which could be removed from the membranes with a weakly acidic buffer, might participate in the infection of HBV. Examination of human serum revealed that β2GPI was a serum protein. Isolation of plasma lipoproteins revealed that β2GPI was in part associated with chylomicrons and high-density lipoproteins, both of which are targeted to hepatocytes during the normal course of lipid metabolism[21]. In this study, we further examined the possible roles of β2GPI in HBV infection. A ligand-blotting system was used to identify and partially characterize a rHBsAg-binding protein associated with hepatocyte membrane, plasma and lipoproteins. Immunoassay was performed to characterize the properties of rHBsAg binding to β2GPI and to demonstrate the specificity of this interaction.
β2GPI is a glycoprotein with four N-linked carbohydrate chains[2,3] present at concentrations of approximately 200 μg/mL in serum[17]. The 326-residue mature protein is composed of a 61 amino acid motif repeated four times, followed by one longer, modified repeat. Each of the first four repeats contains four cysteines in conserved positions, at least one of which is critical for its rHBsAg-binding activity. This pattern of disulfide bonds is similar to the short consensus repeat units found in a family of approximately 20 proteins which includes many of the complement control proteins. It is interesting that the measles virus receptor, CD46[24,25] also belongs to this short consensus repeat family.
The work described in the present report was to reveal the possible roles of β2GPI in HBV infection. As we have known that β2GPI may target to hepatocytes in the form of chylomicrons and high-density lipoproteins. The result of the present paper suggested that β2GPI might specifically bind to HBsAg no matter whether it was under reduced condition or not. We might well reckon that β2GPI might contribute to transportation of HBV to hepatocytes as a kind of carrier. Since β2GPI is associated with lipoproteins, particularly chylomicrons and HDL, it is possible that HBV binds to β2GPI on the surface of these lipoprotein particles. In the bloodstream, chylomicrons are partially degraded by lipoprotein lipase, resulting in chylomicron remnants, which are taken up by hepatocytes. HDLs are also taken up by hepatocytes in the process of “reverse cholesterol transport”. They may have been associated with the process of HBV infection. It is tempted to speculate that infectious HBV might bind to chylomicron or HDL particles by interacting with β2GPI and be taken into hepatocytes as a “hitchhiker” along with these lipoproteins. Our results showed that β2GP I might bind to rHBsAg no matter whether it was under reduced condition or not manifested difference from that reported by Mehdi et al[17]. In Mehdi experimental system, they used human serum to observe the relationship between β2GPI and HBsAg, while we used purified β2GPI. There might exist differences between the two kinds of β2GPI sources in their first, second or third structure, which might lead to the differences of sensitivity to reduced-agents. Moreover, the genetic heterogeneity of β2GPI could also cause mutations of sequences of some domains, which eventually affect the binding of β2GPI to rHBsAg. Related issues should be further investigated.
To sum up, if HBV infection to hepatocytes involves β2GPI on chylomicrons and/or HDL, it is a novel mechanism for virus attachment and entry.