Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.2054
Revised: May 23, 2003
Accepted: June 27, 2003
Published online: September 15, 2003
AIM: To investigate ion channel mechanism in CNP-induced relaxation of gastric circular smooth muscle in guinea pigs.
METHODS: Spontaneous contraction of gastric smooth muscle was recorded by a four-channel physiograph. The whole cell patch-clamp technique was used to record calcium-activated potassium currents and membrane potential in the gastric myocytes isolated by collagenase.
RESULTS: C-type natriuretic peptide (CNP) markedly inhibited the spontaneous contraction in a dose-dependent manner in gastric circular smooth muscle in guinea pigs. Ly83583, an inhibitor of guanylate cyclase, weakened CNP-induced inhibition on spontaneous contraction but Zaparinast, an inhibitor of cGMP sensitive phosphoesterase, potentiated CNP-induced inhibition in gastric circular smooth muscles. The inhibitory effects of CNP on spontaneous contraction were blocked by tetrathylammonium (TEA), a nonselective potassium channel blocker. C N P hyperpolarized membrane potential from -60.0 mV ± 2.0 mV to -68.3 mV ± 3.0 mV in a single gastric myocyte. CNP increased calcium-activated potassium currents (IK(ca)) in a dose-dependent manner in gastric circular myocytes. CNP also increased the spontaneously transient outward currents (STOCs). Ly83583 partly blocked CNP-induced increase of calcium-activated potassium currents, but Zaparinast potented the effect.
CONCLUSION: CNP inhibits spontaneous contraction, and potassium channel may be involved in the process in gastric circular smooth muscle of guinea pigs. CNP-induced increase of IK(ca) is mediated by a cGMP dependent pathway.
- Citation: Guo HS, Cai ZX, Zheng HF, Li XL, Cui YF, Wang ZY, Xu WX, Lee SJ, Kim YC. Role of calcium-activated potassium currents in CNP-induced relaxation of gastric antral circular smooth muscle in guinea pigs. World J Gastroenterol 2003; 9(9): 2054-2059
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/2054.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.2054
Currently, there are six members in the natriuretic peptides (NP) family, including ANP, BNP, CNP, DNP, MNP and VNP. They are distributed in the whole body[1-4], and exhibit natriuresis-diuresis and vasorelaxation, keep electrolyte homeostasis and other qualities. In gastrointestinal tract, the studies mainly focused on absorption, secretion and intestinal motility[5-7]. Our previous study[8,9] indicated that natriuretic peptide receptor (NPR) was distributed in the gastric smooth muscle of rats, and NP inhibited gastric motility in rats, guinea pigs and humans.
There are different views about the mechanism of NP physiological function. Some scholars mentioned that NP did not exert physiological function by cGMP pathway. For example, Carvajal et al[10] indicated that natriuretic peptide-induced relaxation of pregnant myometrium was mediated by a novel mechanism, which was independent of GC-A or GC-B activation, cGMP generation, or clearance receptor activation. However, most scholars supported the views that NP exerted many physiological functions by cyclic guanosine monophosphate (cGMP) and that ion channel participated in the process. McCann et al[11] showed that ANP activated guanylyl cyclase that converted guanosine triphosphate (GTP) into cGMP. cGMP activated protein kinase G (PKG), which regulated Na+ channels to reduce heart rate and the force of contraction, thus decreasing cardiac output. Carini et al[12] demonstrated that ANP enhanced hepatocytic resistance to hypoxia by cGMP-PKC-Na+ channels pathway in rats. Kanwal et al[13] showed that NP regulated release of some neurotransmitters by activating L-type calcium channel in rats. Our previous study demonstrated that CNP significarntly inhibited spontaneous contraction of gastric smooth muscle, and the inhibitory effect was mediated by cGMP pathway in rats[9].
To investigate the ionic channel mechanism in CNP-induced relaxation of gastric circular smooth muscle in guinea pigs, the conventional whole cell patch clamp technique was used to record calcium-activated potassium currents, so that the relationship between IK(ca) and CNP-induced relaxationship could be cleared up.
EWG/B guinea pigs of either sex, weighing (300 ± 50) g bred by Experimental Animal Center of Jilin University, were anaesthetised by a lethal dose of intravenous pentobarbital sodium (50 mg/kg). The abdomen of each rat was opened along the midline and the stomach was removed and placed in a pre-oxygenated Tyrode’s solution at room temperature. The mucous layer was removed. The strips (about 2.0 mm × 15.0 mm) of gastric antral circular and longitudinal smooth muscle were prepared by cutting along the vertical direction of the longer axis of the stomach and along muscle strips were placed in a chamber. One end of the strip was fixed on the lid of the chamber through glass claws, the other end was attached to an isometric force transducer (TD-112S, JAPAN) to record the contraction. The chamber (2 ml volume) was constantly perfused with pre-oxygenated Tyrode’s solution at 1 ml/min. The temperature was maintained at (37.0 ± 0.5) °C by a water bath thermostat (WC/09-05, Chongqing, China). The muscle strips were incubated for at least 40 min before experiments. The samples of human stomach were provided by the Affiliated Hospital of College of Medicine, Yanbian University, (As the gastric sample was limited, the gastric antral of humans was used).
Guinea pigs of either sex, weighing 250-350 g, were anaesthetised by a lethal dose of intravenous pentobarbital sodium (50 mg/kg). The antral part of the stomach was rapidly cut, then the muscosal layer was separated from the muscle layers. The longitudinal layer of muscle was then dissected from the other muscle layers using fine scissors, and then cut into small segments (1 mm × 4 mm). These segments were kept in the modified Kraft-Bruhe (K-B) medium at 4 °C for 15 min. Then, they were incubated at 36 °C in 4 ml of digestion medium [Ca-free physiologic salt solution (Ca-free PSS)] containing 0.1% collagenase II, 0.1% dithioerythreitol, 0.15% trypsin inhibitor and 0.2% bovine serum albumin for 25-35 min. The softened muscle segments were transferred into the modified K-B medium, and the single cells were dispersed by gentle trituration with a wide-bore fire-polished glass pipette. Then, isolated gastric myocytes were kept in a modified K-B medium at 4 °C until they were ready for use.
Isolated cells were transferred to a 0.1 mL chamber on the stage of an inverted microscope (IX-70 Olympus, Japan) and allowed to settle for 10-15 min. Then the cells were continuously perfused with an isosmotic physiologic salt solution at a rate of 0.9-1.0 ml/min. A 8-channel perfusion system (L/M-sps-8, List Electronics, Germany) was used to exchange the solution. The IK(ca) was recorded using the conventional whole cell patch-clamp technique. Patch-clamp pipettes were made from borosilicate glass capillaries (GC 150T-7.5, Clark Electromedical Instruments, UK) using a two-stage puller (PP-83, Narishige, Japan). The resistance of patch pipette was 3-5 MΩ, when it was filled with pipette solution. Liquid junction potentials were cancelled prior to the seal formation. The whole-cell current was recorded with an Axopatch 1-D patch-clamp amplifier (Axon Instrument, USA), and data were filtered at 1 KHz. Command pulses, data acquisition and storage were applied by using the IBM-compatible 486-grade computer and pCLAMP 6.02 software. Spontaneously transient outward currents (STOCs) were recorded simultaneously by EPC-10-HEAKA amplifier (HEAKA Instrument, GERMAN). All experiments were performed at room temperature (20-25 °C).
Tyrode solution containing (mmol·L-1) NaCl 147, KCl 4, MgCl2·6H2O 1.05, CaCl2·2H2O 0.42, Na2PO4. 2H2O 1.81, and 5.5 mM glucose was used. Ca2+-free PSS containing (mmol/L) NaCl 134.8, KCl 4.5, glucose 5, and N-[2-hydroxyethyl] piperazine-N-[2-ethanesulphonic acid] (HEPES) 10 was adjusted to pH7.4 with Tris [hydroxymethyl] aminomethane (TRIZMA). Modified K-B solution containing (mmol/L) L-glutamate 50, KCl 50, taurine 20, KH2PO4 20, MgCl2·6H2O 3, glucose 10, HEPES 10 and egtazic acid 0.5 was adjusted to pH7.40 with KOH. PSS containing (mmol/L) NaCl 134.8, KCl 4.5, MgCl2·6H2O 1, CaCl2·2H2O 2, glucose 5, HEPES 10, sucrose 110, was adjusted to pH7.4 with Tris. CsCl 99, HEPES 10, sucrose 90, were adjusted to pH7.40 with Tris. The pipette solution recording IK(ca) contained (mmol·L-1) potassium-aspartic acid 110, Mg-ATP 5, HEPES 5, MgCl2·6H2O 1.0, KCl 20, egtazic acid 0.1, ditris-creatine phoshate 2.5, disodium-creatine phosphate 2.5 and its pH was adjusted to 7.3 with KOH. Tetraethylammonium (TEA), C-type natriuretic peptide (CNP), Ly83583 and Zaparinast were made as stock solutions. All chemicals in this experiment were purchased from Sigma (USA).
All the data were expressed as means ± SD. Statistical significance was evaluated by a t test. Differences were considered to be significant when P value was less than 0.05.
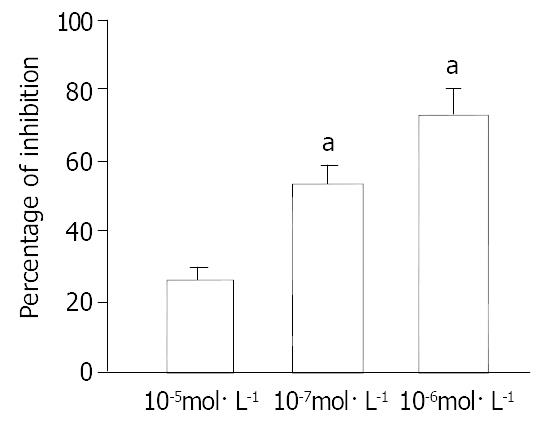
Different concentrations of CNP obviously inhibited spontaneous contraction in a dose-dependent manner and the inhibition percentage was 26.21% ± 3.12%, 52.41% ± 4.21% and 73.42% ± 8.01% (n = 6) at 10-8 mol·L-1, 10-7 mol·L-1 and 10-6 mol·L-1, respectively (Figure 1).
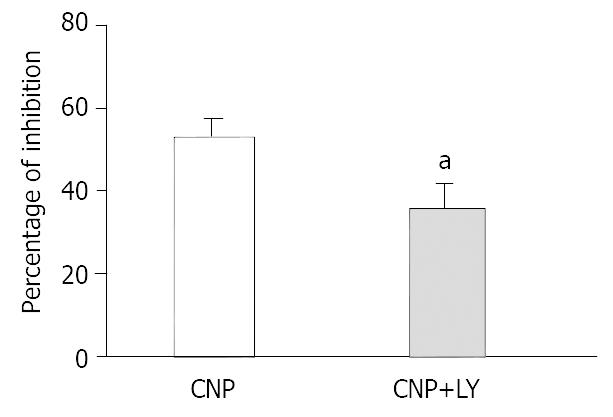
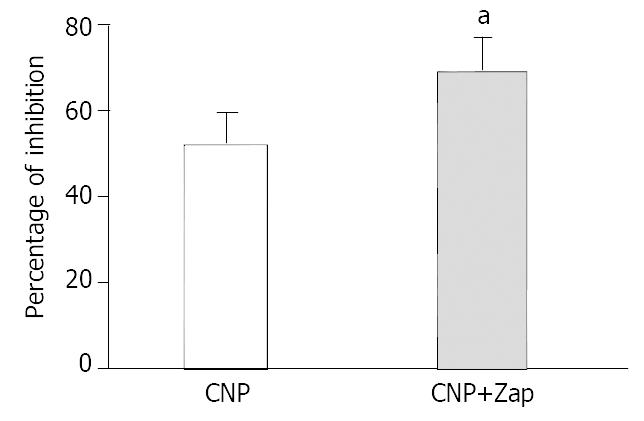
To further investigate the mechanism of CNP-induced inhibition on spontaneous contraction, the effect of CNP on gastric motility was observed in the condition of administering Ly83583, a kind of inhibitor of guanylate cyclase, and Zaparinast as a phosphoesterase inhibitor to change production of cGMP. Ly83583 (10-7 mol·L-1) markedly diminished the inhibitory effect of CNP on spontaneous contraction (P < 0.05) (Figure 2), Zparinast (10-6 mol·L-1) potentiated the inhibitory effect of CNP on spontaneous contraction (P < 0.05) (Figure 3).
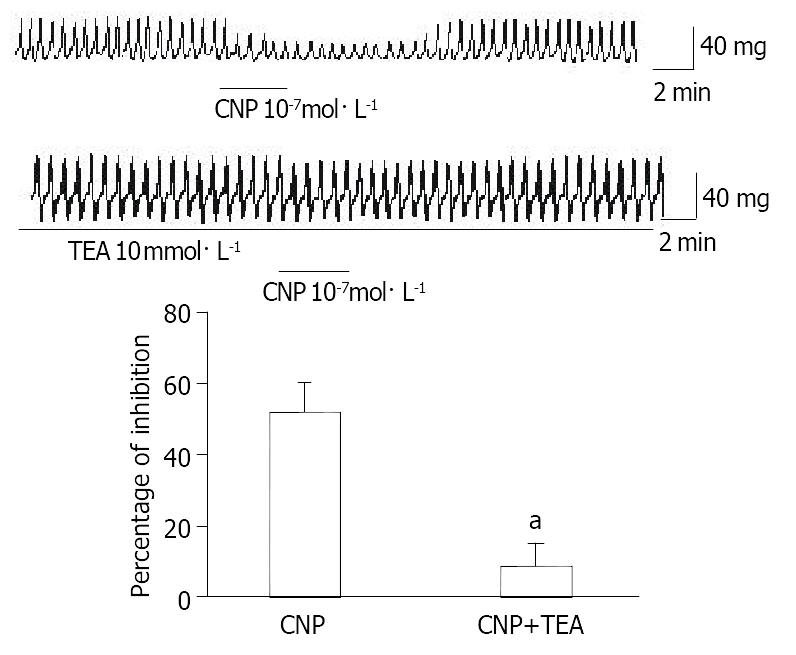
To investigate the relationship between potassium channel and CNP-induced inhibition, the effect of TEA, a nonselective potassium channel blocker, on inhibition of gastic motility induced by CNP was observed. After muscle strips were pretreated with TEA (10 mol·L-1), the inhibitory effect of CNP (10-7 mol·L-1) on spontaneous contraction was significantly diminished (n = 7) (Figure 4).
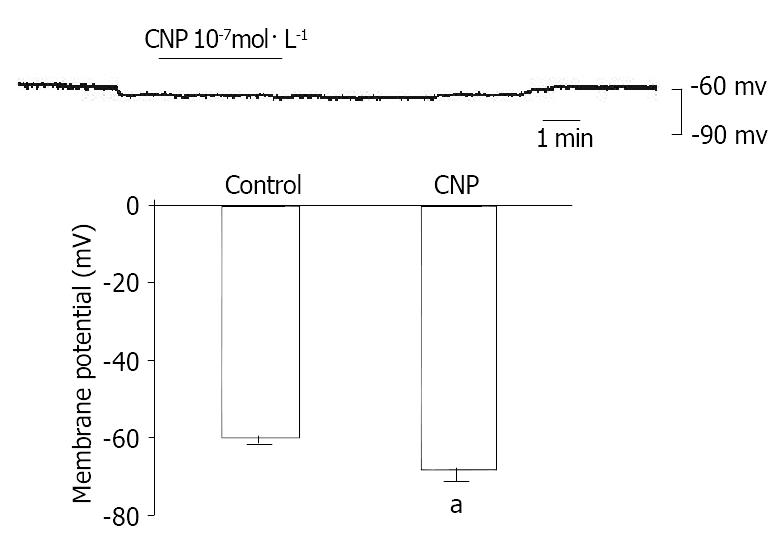
To further investigate the mechanism of CNP-induced relaxation, the conventional whole cell patch-clamp technique was used. Membrane current was clamped and membrane potential was modulated to -60.0 mV, so that it was close to the resting potential of the gastric myocytes. After addtion of CNP(10-7 mol·L-1), the membrane potential was hyperpolarized from -60.0 mV ± 2.0 mV to -68.3 mV ± 3.0 mV, and the amplitude of polarization was increased by 13.31% ± 1.12% (P < 0.05) (Figure 5).
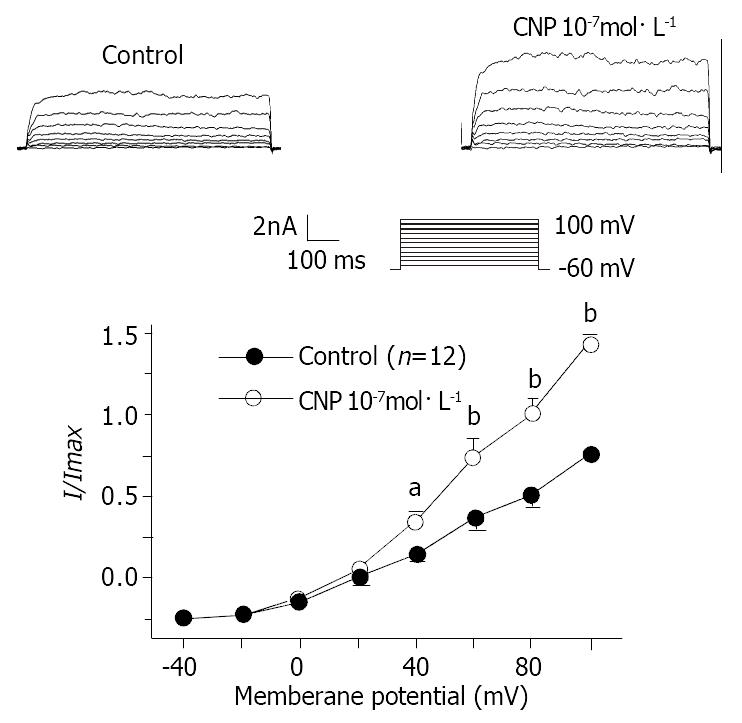
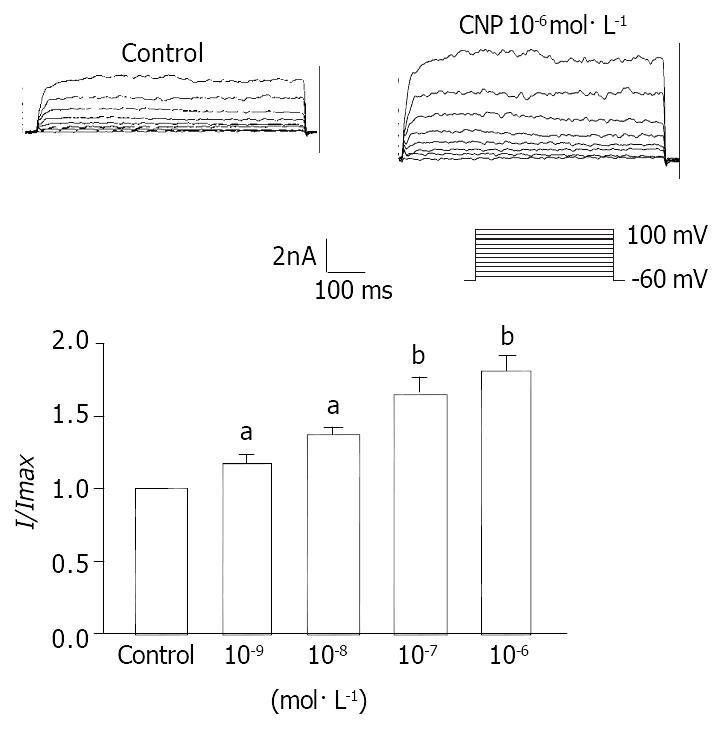
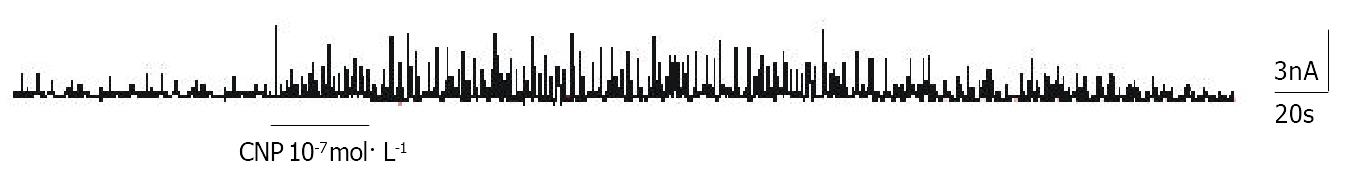
Under conventional whole cell patch clamp mode, the membrane potential was clamped at -60mV, and IK(ca) was elicited by step voltage command pulse from -40 mV to 100 mV for 400 ms with a 20 mV increment at 10 sec intervals. CNP (Figure 6) (10-7 mol·L-1) markedly increased IK(ca) and the increasing amplitude was 62.31% ± 3.22% at 60 mV. Different concentrations of CNP obviously increase IK(ca) in a dose-dependent manner, and the increasing percentage was 16.72% ± 1.12%, 37.51% ± 2.32%, 62.51% ± 3.31% and 82.32% ± 3.71% at 10-9 mol·L-1, 10-8 mol·L-1, 10-7 mol·L-1 and 10-6 mol·L-1 respectively at 60 mV (Figure 7). In conventional whole cell patch clamp mode, the holding potential was clamped at -20 mV, the spontaneously transient outward currents (STOCs) were recorded. CNP (10-7 mol·L-1) also significantly increased STOCs (Figure 8).
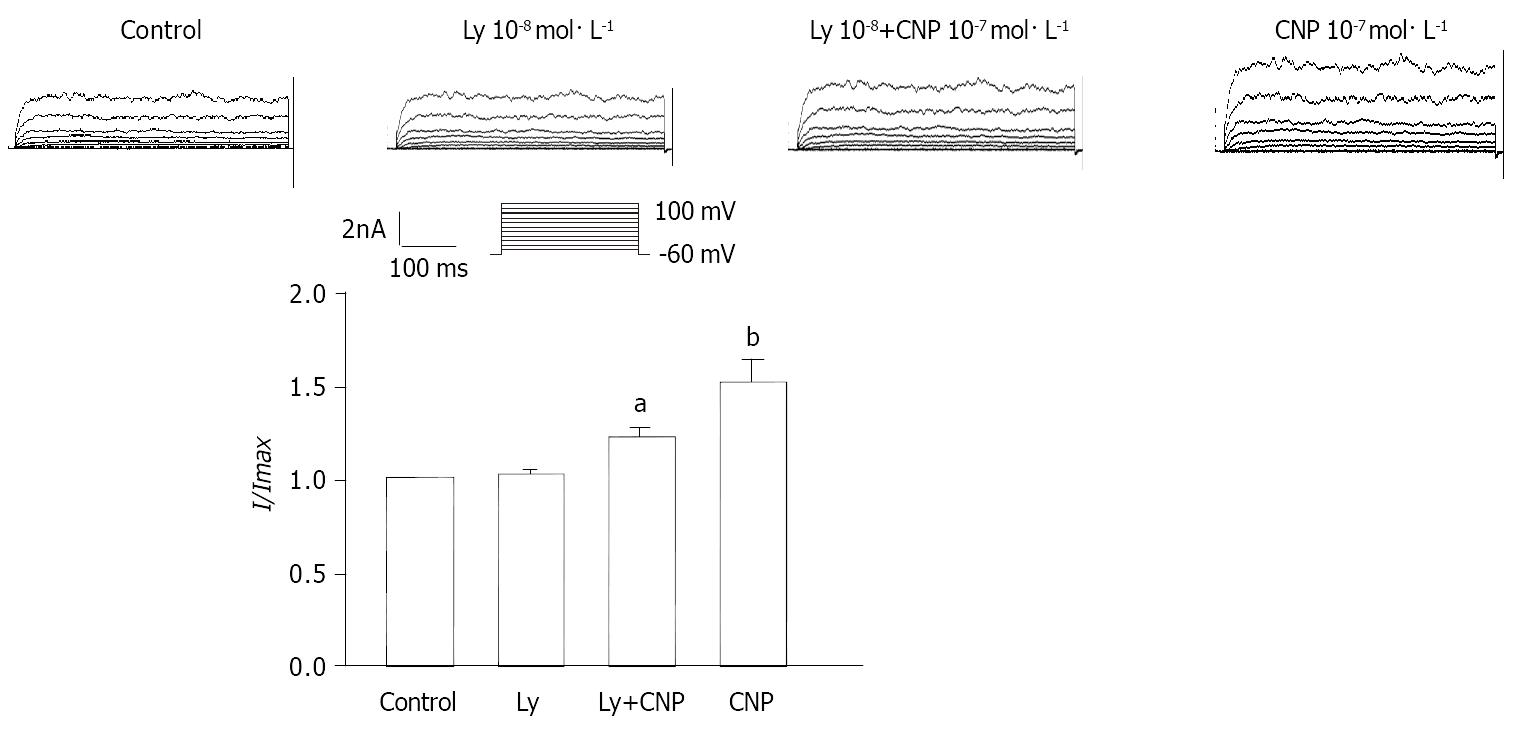
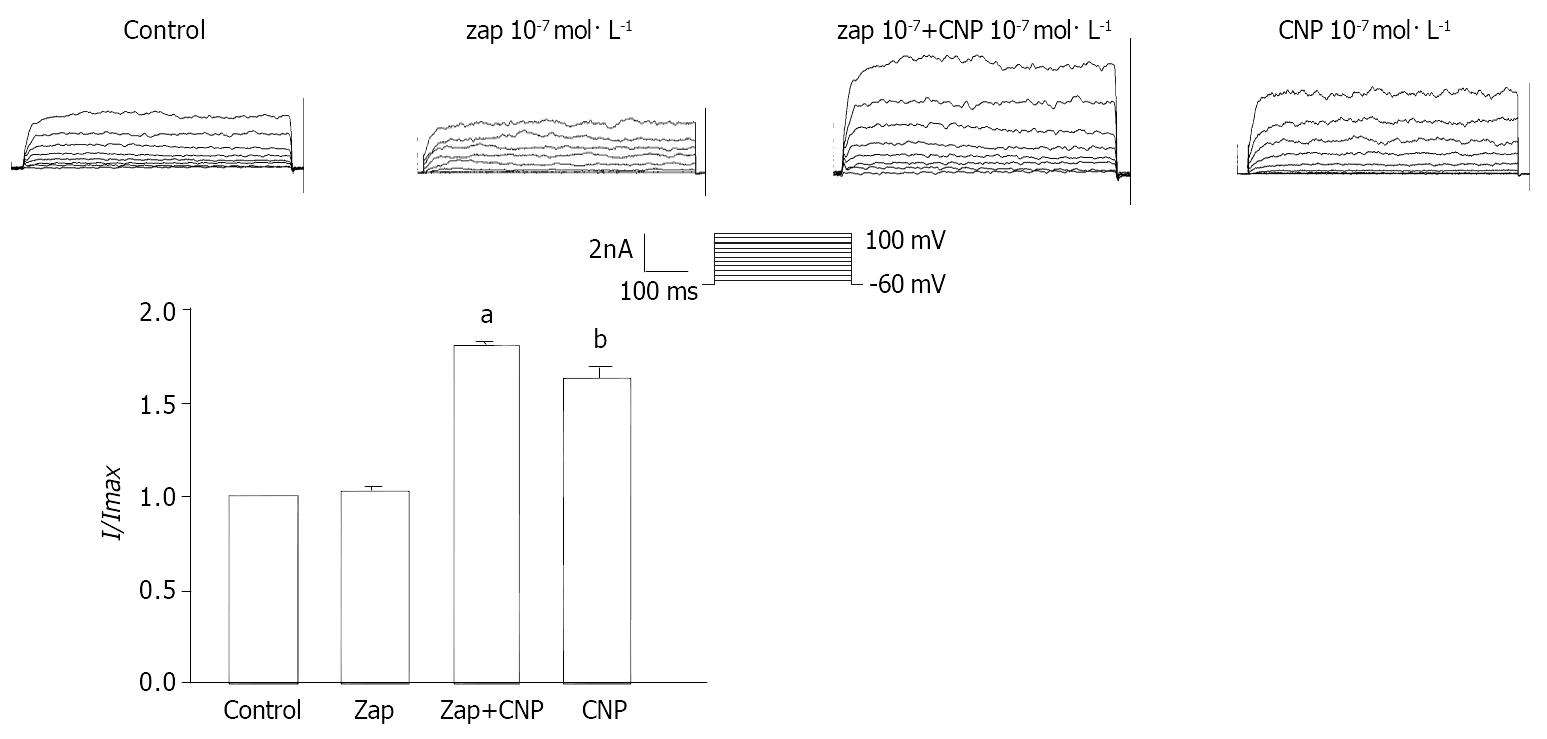
The effect of Ly83583 and Zaparinast on CNP-induced increase of IK(ca) was observed. The percentage of CNP-induced increase was diminished from 64.24% ± 3.32% to 26.53% ± 2.31% at 60 mV after pretreated with Ly83583 (P < 0.05) (Figure 9) at the same time, the percentage of CNP-induced increase was potentiated from 63.71% ± 1.82% to 81.13% ± 2.21% at 60 mV after pretreated with Zaparinast (P < 0.05) (Figure 10).
The present study indicated that CNP inhibited spontaneous contraction of the gastric antral smooth muscle in guinea pigs, and the inhibitory effect was markedly decreased by Ly83583 and potented by Zaparinast. TEA blocked CNP-induced inhibition. CNP hyperpolarized membrane potential and obviously increased IK (ca) in gastric circular myocytes in guinea pigs. CNP-induced increase of IK(ca) was partly blocked by Ly83583, but potentiated by Zaparinast.
Since Komatsu et al[14] demonstrated that CNP was distributed in the gastrointestinal tract of rats and humans, the study on the relationship between CNP and gastrointestinal function has recently become the hot topic. In the present study, CNP inhibited spontaneous contraction of gastric circular smooth muscle in guinea pigs. It was similar to many previous studies. Kim et al[15,16] indicated that CNP inhibited colonic motility of rabbits and both the frequency and the amplitude of basal motility of the oviduct in a dose-dependent manner. Our previous study also demonstrated that CNP inhibited spontaneous contraction of gastric circular smooth muscles in rats, guinea pigs and humans[8].
In this study, CNP-induced relaxation was mediated by cGMP pathway, since Ly83583 markedly diminished CNP-induced inhibition on spontaneous contraction, but Zaparinast obviously potentiated the effects in the gastric antral circular smooth muscle of guinea pigs. Many studies demonstrated that NP exerted a physiological function by cGMP pathway. Wellard et al[17] showed that atrial natriuretic peptides elevated cGMP levels in the primary cultures of rat ependymal cells. ANP and endothelin-1 (ET-1) might prevent renal dysfunction during the progression of congestive heart failure (CHF) through the cGMP pathway in dogs[18]. Tsuruda et al[19] demonstrated that BNP could control cardiac fibroblast function via cGMP pathway.
In our present study, TEA weakened the effect of CNP-induced inhibition on spontaneous contraction and CNP hyperpolarized membrane potential in gastric myocytes. It is well known that potassium channel has an intimate relationship with relaxation of smooth muscles. There were two kinds of potassium current, calcium-activated potassium current and delayed rectified potassium current in gastric antral smooth musle cells of guinea pigs[20,21]. This study demonstrated that CNP increased IK(ca) in a dose-dependent manner and CNP also increased STOCs. It could be concluded that potassium channel participates in CNP-induced relaxation. Many previous studies also reported that potassium channel was involved in NP-induced regulation of many physiological functions. Van der Zander et al[22] demonstrated that NO and calcium-activated potassium currents could regulate the effect of BNP-induced relaxation on vascular smooth muscles. In guinea pig sino-atrial (SA) node cells, ANP and cGMP increased the delayed rectified potassium current to exert certain physiological functions[23]. However, CNP relaxed the coronary arterial smooth muscle by activating of low conductance Ca2+-activated K+ channels, natriuretic peptide clearance receptors, and activity/regulation of phosphodiesterases in pigs[24].
NP can exert physiological functions by cGMP-PKG-ion channel pathway. Nakamura et al[25] indicated that ANP enhanced the level of cGMP to active cGMP-dependent protein kinase (PKG) to change the activity of inwardly rectifying K(+) channel. Nara et al[26] demenstrated that ANP increased IK(ca) by cGMP-PKG pathway in Xenopus oocytes. Our next step is to determine the relationship between CNP-induced increase of IK(ca) and cGMP-PKG pathway in gastric circular myocytes of guinea pigs.
In summary, CNP activated IK(ca) by cGMP pathway can hyperpolarize membrane potential, and gastric smooth muscle can be relaxed in guineapigs.
Edited by Zhang JZ and Wang XL
| 1. | Lai FJ, Hsieh MC, Hsin SC, Lin SR, Guh JY, Chen HC, Shin SJ. The cellular localization of increased atrial natriuretic peptide mRNA and immunoreactivity in diabetic rat kidneys. J Histochem Cytochem. 2002;50:1501-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Cayli S, Ustünel I, Celik-Ozenci C, Korgun ET, Demir R. Distribution patterns of PCNA and ANP in perinatal stages of the developing rat heart. Acta Histochem. 2002;104:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic Peptide activity. Circulation. 2003;107:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Peng N, Chambless BD, Oparil S, Wyss JM. Alpha2A-adrenergic receptors mediate sympathoinhibitory responses to atrial natriuretic peptide in the mouse anterior hypothalamic nucleus. Hypertension. 2003;41:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Vuolteenaho O, Arjamaa O, Vakkuri O, Maksniemi T, Nikkilä L, Kangas J, Puurunen J, Ruskoaho H, Leppäluoto J. Atrial natriuretic peptide (ANP) in rat gastrointestinal tract. FEBS Lett. 1988;233:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Brockway PD, Hardin JA, Gall DG. Intestinal secretory response to atrial natriuretic peptide during postnatal development in the rabbit. Biol Neonate. 1996;69:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Akiho H, Chijiiwa Y, Okabe H, Harada N, Nawata H. Interaction between atrial natriuretic peptide and vasoactive intestinal peptide in guinea pig cecal smooth muscle. Gastroenterology. 1995;109:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Guo HS, Jin Z, Jin ZY, Li ZH, Cui YF, Wang ZY, Xu WX. Comparative study in the effect of C-type natriuretic peptide on gastric motility in various animals. World J Gastroenterol. 2003;9:547-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Guo HS, Cui X, Cui YG, Kim SZ, Cho KW, Li ZL, Xu WX. Inhibitory effect of C-type natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscle of rat. Acta Pharmacol Sin. 2003;24:1021-1026. [PubMed] |
| 10. | Carvajal JA, Aguan K, Thompson LP, Buhimschi IA, Weiner CP. Natriuretic peptide-induced relaxation of myometrium from the pregnant guinea pig is not mediated by guanylate cyclase activation. J Pharmacol Exp Ther. 2001;297:181-188. [PubMed] |
| 11. | McCann SM, Gutkowska J, Antunes-Rodrigues J. Neuroendocrine control of body fluid homeostasis. Braz J Med Biol Res. 2003;36:165-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Carini R, De Cesaris MG, Splendore R, Domenicotti C, Nitti MP, Pronzato MA, Albano E. Mechanisms of hepatocyte protection against hypoxic injury by atrial natriuretic peptide. Hepatology. 2003;37:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kanwal S, Elmquist BJ, Trachte GJ. Atrial natriuretic peptide inhibits evoked catecholamine release by altering sensitivity to calcium. J Pharmacol Exp Ther. 1997;283:426-433. [PubMed] |
| 14. | Komatsu Y, Nakao K, Suga S, Ogawa Y, Mukoyama M, Arai H, Shirakami G, Hosoda K, Nakagawa O, Hama N. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology. 1991;129:1104-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Kim JH, Jeon GJ, Kim SZ, Cho KW, Kim SH. C-type natriuretic peptide system in rabbit colon. Peptides. 2001;22:2061-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kim SH, Lee KS, Lee SJ, Seul KH, Kim SZ, Cho KW. C-type natriuretic peptide system in rabbit oviduct. Peptides. 2001;22:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Wellard J, Rapp M, Hamprecht B, Verleysdonk S. Atrial natriuretic peptides elevate cyclic GMP levels in primary cultures of rat ependymal cells. Neurochem Res. 2003;28:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Yamamoto T, Wada A, Ohnishi M, Tsutamoto T, Fujii M, Matsumoto T, Takayama T, Wang X, Kurokawa K, Kinoshita M. Chronic administration of phosphodiesterase type 5 inhibitor suppresses renal production of endothelin-1 in dogs with congestive heart failure. Clin Sci (Lond). 2002;103 Suppl 48:258S-262S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Li Y, Xu WX, Li ZL. Effects of nitroprusside, 3-morpholino-sydnonimine, and spermine on calcium-sensitive potassium currents in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin. 2000;21:571-576. [PubMed] |
| 21. | Piao L, Li Y, Li L, Xu WX. Increment of calcium-activated and delayed rectifier potassium current by hyposmotic swelling in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin. 2001;22:566-572. [PubMed] |
| 22. | van der Zander K, Houben AJ, Kroon AA, De Mey JG, Smits PA, de Leeuw PW. Nitric oxide and potassium channels are involved in brain natriuretic peptide induced vasodilatation in man. J Hypertens. 2002;20:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Shimizu K, Shintani Y, Ding WG, Matsuura H, Bamba T. Potentiation of slow component of delayed rectifier K(+) current by cGMP via two distinct mechanisms: inhibition of phosphodiesterase 3 and activation of protein kinase G. Br J Pharmacol. 2002;137:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Barber DA, Burnett JC, Fitzpatrick LA, Sieck GC, Miller VM. Gender and relaxation to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol. 1998;32:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Nakamura K, Hirano J, Itazawa S, Kubokawa M. Protein kinase G activates inwardly rectifying K(+) channel in cultured human proximal tubule cells. Am J Physiol Renal Physiol. 2002;283:F784-F791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Nara M, Dhulipala PD, Ji GJ, Kamasani UR, Wang YX, Matalon S, Kotlikoff MI. Guanylyl cyclase stimulatory coupling to K(Ca) channels. Am J Physiol Cell Physiol. 2000;279:C1938-C1945. [PubMed] |