Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.2030
Revised: April 23, 2003
Accepted: May 24, 2003
Published online: September 15, 2003
AIM: To investigate the anti-tumor mechanism of antisense oligodeoxynucleotide cantide against hTERT.
METHODS: Tumor cells were cultured overnight and grown to 50%-60% confluence. HepG2 and SMMC-7721 were treated with cantide mixed with lipofectin, or lipofectin alone. After inducted for 6 h at 37 °C, 10% FCS in DMEM was replaced in each well. After the treatment repeated twice to three times in each concentration of cantide, hTERT mRNA and protein expression were measured by RT-PCR and Western blot analysis, respectively. Telomerase activity was determined by TRAP-ELISA assay. CPP32- and ICE-like activity was also investigated using CasPACE assay system at 48 h after cantide treatment, and apoptosis was evaluated using the DeadEnd assay at 24, 48 and 72 h after cantide treatment.
RESULTS: Compared to the control cells, the cells treated with cantide showed a dose-dependent decrease in hTERT mRNA levels at 24 h and in protein levels at 48 h respectively. The telomerase activity was decreased as the concentration of cantide increased at 48 h. At the concentration of 800 nM, the telomerase activity in the treated HepG2 and SMMC-7721 cells was only 17.1% (P < 0.01) and 20.3% (P < 0.01) of that in untreated cells. The levels of CPP32-like protease activity in HepG2 and SMMC-7721 increased by 2.8- and 3.0-fold (P < 0.05) at 48 h, and the levels of ICE-like protease activity also increased by 2.6- and 3.2-fold (P < 0.05) respectively. The percentage of apoptosis in HepG2 and SMMC-7721 cells treated with 800 nM cantide at 72 h was 63% and 52% (P < 0.01), respectively. By contrast, 8% and 9% of the cells were apoptosis after 72 h treatment with lipofectin alone.
CONCLUSION: Cantide can decrease telomerase activity by inhibiting the expression of hTERT gene and has a rapid anti-tumor effect through inducing the Caspase-dependent apoptosis. The rapid inhibitory effect of cantide on tumor growth demonstrates its feasibility in cancer treatment.
- Citation: Du QY, Wang XB, Chen XJ, Zheng W, Wang SQ. Antitumor mechanism of antisense cantide targeting human telomerase reverse transcriptase. World J Gastroenterol 2003; 9(9): 2030-2035
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/2030.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.2030
Telomerase is a unique ribonucleoprotein enzyme responsible for adding the telomeric repeats onto the 3’ ends of chromosome[1]. Telomerase plays an important role in the development of cellular immortality and oncogenesis[2,3]. Previous studies have shown that telomerase activity is found in 85%-90% of all human tumors, but not in their adjacent normal cells[4,5]. This makes telomerase a good target not only for cancer diagnosis, but also for the development of novel therapeutic agents[6,7].
The research of antisense oligodeoxynucleotides (ODN) is an area of heightened interest in the field of telomerase inhibition[8]. Antisense ODNs have been investigated on the inhibition of telomerase and suppression of tumor growth. But most of these antisense ODNs were designed to target the hTR template, and they did not reduce telomerase activity and tumor growth effectively[9-12]. It has been shown that expression of hTERT is closely associated with telomerase activity in human tumor cells while all human somatic cells constitutively contain hTR. Therefore, to significantly inhibit telomerase activity, hTERT might be more attractive as a target than hTR[13,14]. A series of antisense oligonucleotides were designed based on hTERT mRNA secondary structure. It has been demonstrated that an antisense oligonucleotide cantide has a strong inhibitory effect on tumor cell growth. The cytotoxic effect of a specific cantide antisense was also assessed by using the sense, random and mismatched ODN, only cantide had potent inhibitory effect on proliferation of tumor cells[15], and in vivo treatment of HepG2 tumor xenografts with cantide antisense ODN significantly retarded the growth of these tumors (data unpublished). To investigate the possible mechanism of antitumor effect of cantide, an in vitro study was performed in this report.
Human hepatocellular carcinoma cells (HepG2, SMMC-7721) were obtained from Chinese National Cancer Institute, Chinese Academy of Medical Sciences, Beijing. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (GIBCO BRL, Grand Island, NY), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C with 5% CO2.
The following specific PCR primers were synthesized by an applied biosystems 391 DNA synthesizer and purified by OPC (Perkin-Elmer, Foster city, CA). For β2-microglobulin, sense (5’-TTCAGGTTTACTCACGTCATCC-3’) and antisense (5’-CCAAATGCGGCATCTTCAAACCC-3’), amplification of 317 bp DNA fragment. For hTERT, sense (5’-TCTACCGGAAGAGTGTCTGGAGCAA-3’) and antisense (5’-GCGCCCACGACGTAGTCCATGTTCA-3’), amplification of 202 bp DNA fragment. The selection of antisense ODNs against hTERT was described previously[15]. The sequence of the cantide was 5’-ACTCACTCAGGCCTCAGACT-3’. The phosphorothioate cantide was synthesized on solid supports using Oligo Pilot II DNA (Amersham-Pharmacia, Piscataway, NJ) and purified by HPLC (Waters Delta Prep 4000) with SOURCE 15Q (Amersham-Pharmacia), and the purity of cantide was over 95%.
A total of 1.5 × 105 cells were seeded in a 6-well plate, and treated with 50, 100, 200, 400 or 800 nM cantide respectively mixed with 4 μL lipofectin (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The first day of treatment was designated as day 0. After 24 h, total RNA was isolated using TRIZOL (Invitrogen) by a single-step phenol-extraction method as templates. Subsequent RT-PCR reaction was performed using a reverse transcription system (RT-PCR kit, Promega, Madison, WI.). Briefly, first strand cDNA was synthesized using a Oligo (dT)15 primer at 42 °C for 30 min. PCR reaction for hTERT and β2-microglobulin was performed in a single reaction of 20 μL volume. The latter served as a control following 28 cycles of denaturing at 95 °C for 45 s, annealing at 58 °C for 40 s, and extending at 72 °C for 40 s. At this PCR condition, the amplification showed linearity as was determined experimentally (data not shown). PCR products were run on a 2.0% agarose gel and visualized by ethidium bromide staining, and the intensities were then measured by scanning the gel with Gel Doc 1000 (Bio-Rad, Hercules, CA). Inhibition of hTERT mRNA was calculated by normalized intensity ratio hTERT: β2-microglobulin of control sample according to the following formula:
Inhibition percentage (%) = [1 - (Asample× A0 control)/(Acontrol× A0 sample)] × 100
Asample: the intensity of hTERT PCR product in cells treated with cantide and lipofectin, A0 sample: the intensity of hTERT PCR product in cells treated with lipofectin alone, Acontrol: the intensity of β-actin product in cells treated with cantide and lipofectin, A0 control, the intensity of β-actin product in cells treated with lipofectin alone.
The levels of hTERT protein in cells treated with cantide were measured by scanning the density of bands on Western blot. The treatment of cantide was performed using the same method as for the hTERT mRNA level analysis described above. After 48 h of transfection, cells were collected by trypsinization and lysed in a lysis buffer containing 9.1 mM Na2HPO4, 1.7 mM NaH2PO4 (pH7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/mL PMSF, 1 μg/mL aprotinin and 1 mM sodium orthovanadate for 30 min on ice. Equal amount (60 μg) of proteins was run on a 6% SDS-PAGE gel, and transferred onto Hybond-polyvinylidene difluoride membranes (Amersham, Arlington Heights, IL). The transferred membrane was incubated first with primary antibodies against hTERT (Santa Cruz Biotechnology, Santa Cruz, CA) or primary antibodies against actin, and followed with peroxidase-linked secondary antibody, and finally the ECL Western blot system (Promega) was used for developing the bands. The intensity of the bands was scanned by Gel Doc 1000 (Bio-Rad), and the inhibition percentage (%) was calculated according to the following formula: inhibition percent = (1 - Asample/Acontrol) × 100.
TRAP-ELISA was performed using the TeloTAGGG and Telomerase PCR ELISA kit (Roche, Indianapolis, IN) following the manufacturer’s instructions. Telomerase levels were determined after 48 h treatment of cantide (400 nM) transfected using the method as for the hTERT mRNA level analysis described above. These cells were grown in 24-well plates. Cells were homogenized in 200 μL lysis buffer and incubated on ice for 30 min. To determine the specificity of the assay, all protein samples were preheated for 10 min at 70 °C to inactivate telomerase and tested in parallel in the assay. 5 μg of each extract was assayed in 50 μL reaction mixture containing 25 μL of reaction mixture and 5 μL of the internal standard. After incubated at 25 °C for 30 min, the reaction mixture was then subjected to PCR amplification in a thermal cycler for 20 cycles at 94 °C for 30 s, at 50 °C for 30 s, and at 72 °C for 90 s. Subsequently, ELISA reaction was performed following the manufacturer’s instructions.
Apoptosis of treated tumor cells was detected using TUNEL technique. Cells (5 × 103/well) were plated in 96-well dishes and treated with cantide at a final concentration of 400 nM. At 24, 48, and 72 h after transfection, modified TUNEL assay was performed using DeadEnd colorimetric apoptosis detection system (Promega) according to the manufacturer’s instructions. Briefly, cells were harvested, washed and fixed on silanized microscope slides. Biotinylated nucleotide was incorporated at the 3’-OH DNA ends using terminal deoxynucleotidyl transferase (TdT). Streptavidin-HRP was then bound to these biotinylated nucleotides, which were detected using hydrogen peroxide and diaminobenzidine (DAB). With this procedure, apoptotic cell nuclei were stained dark brown. Stained cells were observed under a light microscope.
Caspase (CPP32-like and ICE-like) activity was evaluated using CaspACE assay system fluorometry (Promega) according to the protocol provided by the manufacturer. Cells were plated in flasks and treated with 400 nM cantide mixed with lipofectin. At 48 h after cantide treatment, cells were harvested and washed, lysed by subjecting to four cycles of freezing and thawing, and centrifuged at 16000 g for 20 min at 4 °C. The same amount of supernatant was pipetted into a 96-well plate, then the following items were added into each well, namely 32 μL buffer, 2 μL DMSO, 100 μL DTT (100 mM), 2 μL of appropriate substrate (2.5 mM). The final volume was 100 μL. Blank (without cells extracts) and negative control (with inhibitor) reaction mixtures were also prepared simultaneously. After incubated at 30 °C for 60 min, the fluorescence of the reactions at an excitation wavelength of 355 nm and emission wavelength of 460 nm was measured. Caspase activity was determined in duplicate cells. Relative fluorescence units (△FU) were calculated according to the formula: △FU = (FUassay - FUblank) - (FUnegative - FUblank).
The data were expressed as means ± standard deviation (SD), statistical analysis was performed by Student’s-t-test (two-tailed).All data represented at least two independent experiments.
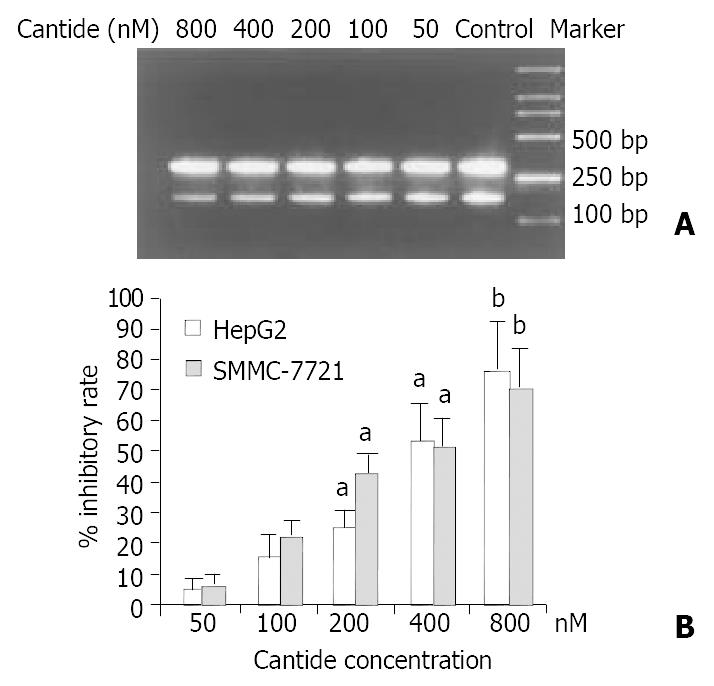
It has been suggested antisense ODNs may inhibit gene expression through diverse effects on transcription and translation, inhibition of mRNA transcription can occur through formation of triple helixes with complementary regions in DNA, and/or mRNA can be cleaved by recruitment of the endogenous nuclease RNase-H to catalyze the degradation of ODN-RNA dimmers[16]. Therefore, the mRNA level of hTERT was determined by semi-quantitative RT-PCR. A 202 bp DNA fragment of hTERT gene and a 317 bp DNA fragment of β2-microglobulin gene were amplified by RT-PCR with specific primers, respectively. Figure 1A shows that the mRNA expression level of hTERT was decreased as the concentration of cantide treatment increased at 24 h when compared to the untreated cells, while the mRNA level of β2-microglobulin as a control was almost unchanged. As shown in Figure 1B, after treatment with 800 nM cantide normalized according to the levels of β2-microglobulin, the relative inhibition rate of hTERT mRNA expression was 74.4% (P < 0.01) and 68.4% (P < 0.01) in treated tumor cells HepG2 and SMMC-7721, respectively.
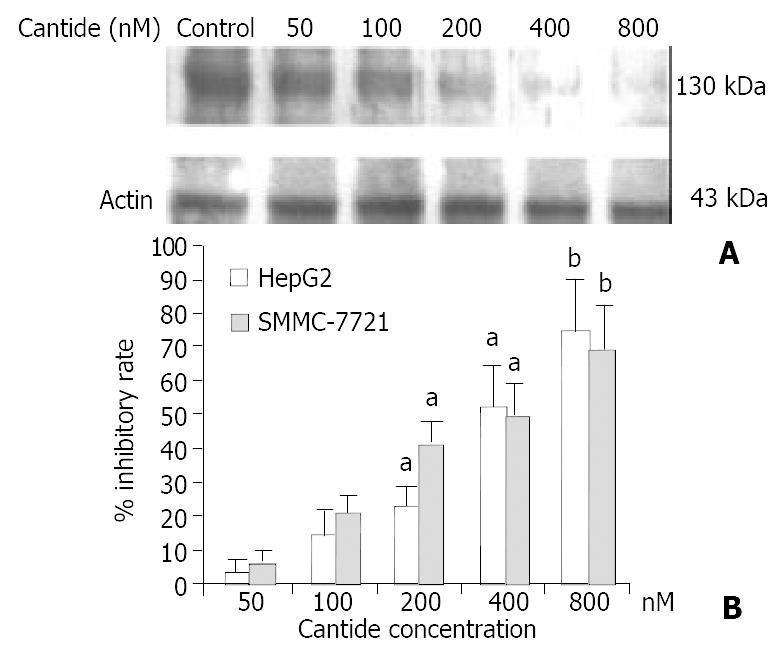
Western blot analysis was performed to determine the effect of cantide treatment on hTERT protein level in tumor cells. Figure 2A shows that the protein level of hTERT was decreased as the concentration of cantide increased at 48 h compared to the untreated cells. The relative inhibition percentage of hTERT protein was 67.8% (P < 0.01) and 66.2% (P < 0.01) in HepG2 and SMMC-7721 cells treated with 800 nM cantide, respectively (Figure 2B).
These results indicated that cantide inhibited hTERT gene expression in a dose-dependent manner after 48 h treatment.
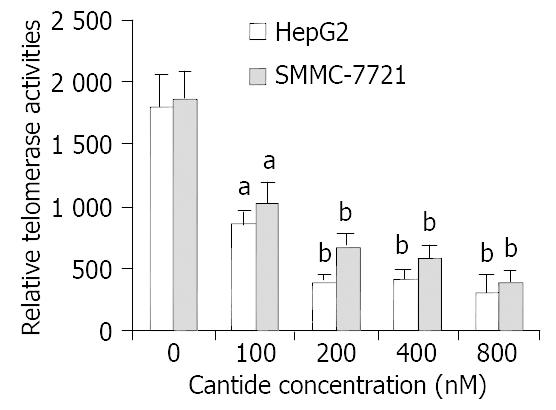
It was shown that the expression of hTERT gene appeared to correlate with the level of telomerase enzymatic activity in many human tumor cells[17,18]. To investigate whether the telomerase activity was affected in tumor cells treated with cantide, a TRAP-ELISA assay was performed. Figure 3 shows that the decrease of telomerase activity was observed after 48 h of treatment at various concentrations of cantide. Moreover, the decrease of telomerase activity was approximately correlated with the concentration of cantide. At the concentration of 800 nM, the relative telomerase activity in the treated HepG2 and SMMC-7721 cells was only 17.1% (P < 0.01) and 20.3% (P < 0.01) of that in untreated cells at 48 h, respectively. These findings suggested that treatment of cantide decreased the telomerase activity in a dose-dependent manner by direct inhibition of the hTERT gene expression.
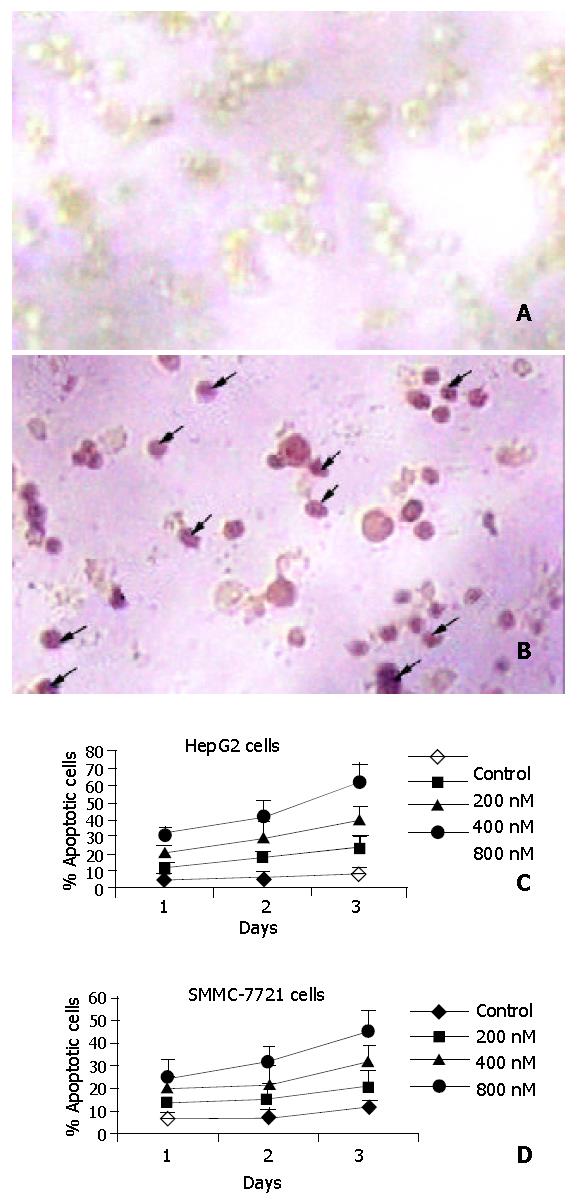
The DeadEnd colorimetric apoptosis detection system labeled fragmented DNA in situ and was used for detecting apoptosis, which was used to investigate whether cantide induced apoptosis in tumor cells. Figure 4A shows the staining of HepG2 cells 72 h after treatment. The cells treated with lipofectin alone showed very few stained cells (Figure 4 Aa). In contrast, most of the cells treated with cantide were intensively stained (Figure 4 Ab). To quantify the extent of apoptosis, the percentage of stained cells from a total of 200 cells was determined in each treatment group. As shown in Figure 4B, the percentage of apoptotic cells after cantide treatment was increased both in a time-dependent manner and in a dose-dependent manner. The percentage of stained cells in HepG2 and SMMC-7721 cells treated with 800 nM cantide at 72 h was 63% and 52% (P < 0.01), respectively. By contrast, 8% and 9% cells were stained after 72 h treatment with lipofectin alone. These results suggested that the cytotoxic effect of cantide was mainly due to induction of apoptosis.
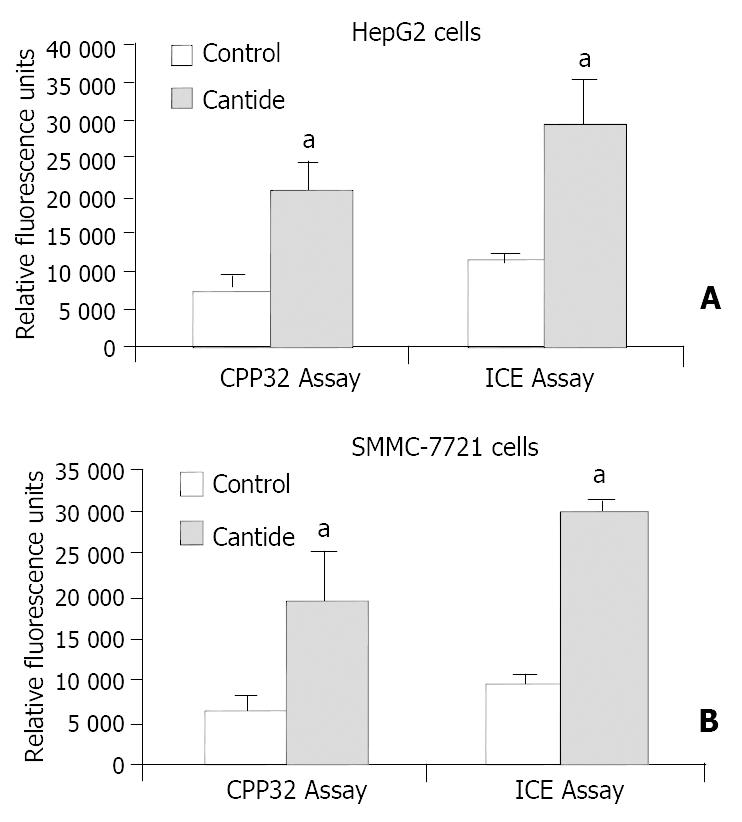
It was shown that Caspases were the main factor in the apoptotic pathway[19,20]. We investigated whether Caspase was involved in the anti-tumor effect by utilizing a Caspase activity detection assay. We observed that ICE- and CPP32-like protease activity in tumor cell treated with cantide reached the highest point at 48 h (data not shown). The levels of CPP32-like protease activity in HepG2 and SMMC-7721 were increased 2.8- and 3.0-fold respectively at 48 h (P < 0.05) compared to the cells treated with lipofectin alone, and the levels of ICE-like protease activity were also increased 2.6- and 3.2-fold (P < 0.05) at 48 h, respectively (Figure 5). These results clearly indicated the involvement of Caspases family (at least ICE- and CPP-like) in the induction of apoptosis by cantide treatment.
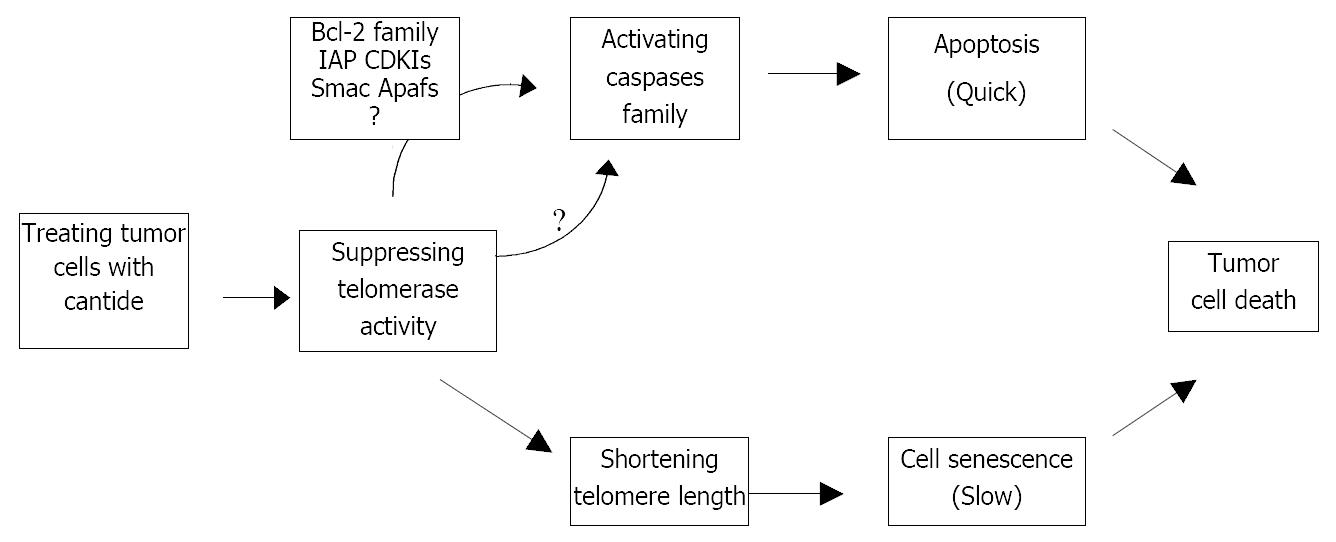
The hypothesis of telomerase mechanism is supported by the idea that progressive shortening of telomeres regulates the lifespan of cells, with each cell division, telomeres are shortened by 50-200 bp.When telomeres become critically short, the cells turn to growth arrest[21,22]. The rate of telomere DNA shortening is regulated by telomerase expression and activity[23]. Therefore, telomerase inhibitors might be useful as anticancer agents, but there will be an expected log phase between the time when telomerase is inhibited and the time when telomere of cancer cells is shortened sufficiently to produce detrimental effects on cell proliferation[24]. It takes approximately 1 month to induce cell death in tumor cells following telomerase inhibition by transfection of antisense hTR vector[25]. Our previous study has shown that cantide has a rapid inhibitory effect on tumor growth 3 days after in vitro treatment[15], and this striking effect is unlikely to be interpreted by the hypothesis that cantide is acting specifically through a telomere-dependent mechanism because the cells have not undergone sufficient cell divisions to significantly shorten their telomeres. It is likely that there might be other mechanisms for the antitumor effect of cantide.
In this study, we detected apoptosis in tumor cells just 3 days after cantide treatment in a dose-dependent manner. Taking previous reports on antisense hTR vector and our studies together into consideration, it is likely to raise the possibility that antitumor effect of cantide occurs through following two pathways: 1) A short-term effect on apoptosis is induced rapidly by cantide. 2) A long-term effect on telomerase activity is inhibited, and cell death is caused when telomere length is critically shortened by telomeric DNA.
It has been demonstrated that Caspase is a central executioner of apoptosis machinery, and over-expression and activation of Caspases in human cells lead to apoptosis[26,27]. To date, fourteen mammalian Caspases have been described. Among them, two subfamilies have been categorized based on amino acid sequence, substrate and inhibitor specificities. They are ICE-like proteases and CPP32-like proteases[28]. In this study, we found that both CPP32- and ICE-like proteases were increased 2 or 3-fold in tumor cells compared to the control cells 2 days after cantide treatment. Based on this evidence, the rapid antitumor effect of cantide may be due to active induction of Caspase-dependent apoptosis. Cantide can activate Caspase activity and lead to rapid cell death via apoptotic pathways, and might be a potential therapeutic agent for the treatment of cancer.
It has been found that 2-5A antisense ODN could cause profound cell death in prostate cancer cells and ovarian cancer cells, but not in fibroblast cells and normal ovarian epithelial cells without telomerase activity[12,29]. Furthermore, suppression of TERT levels and function in embryonic mouse hippocampal neurons in culture could significantly increase their vulnerability to cell death induced by amyloid beta-peptide, and over-expression of TERT in pheochromocytoma cells could result in decrease of vulnerability to amyloid beta-peptide-induced apoptosis[13]. In addition, hTERT gene could be transfected into telomerase negative human embryo lung fibroblasts, the telomerase-expression cells could elongate telomeres and increase resistance to apoptosis induced by hydroxyl radicals[30]. We detected the down-regulation of hTERT mRNA gene expression, and found a significant decrease in telomerase activity after cantide treatment. The decrease was correlated with the concentration of cantide. Based on the observation above, we speculate that telomerase plays another role in addition to maintaining the telomere length, disturbance of this function will cause a rapid cell death by activating the Caspases. In accordance to that hypothesis, Cao et al[31] have also reported that telomerase plays roles not only in up-regulating cell proliferative life span, but also in supporting cell proliferative rate by a mechanism involving telomere lengthening-independent activity. Therefore, there should be some proteins that deliver messages between telomerase and Caspase, and further studies using techniques such as biochip or yeast two-hybrid system are necessary to find certain associated molecules.
In summary, our results have shown that cantide can effectively inhibit hTERT gene expression, decrease telomerase activity, and trigger apoptosis through activation of Caspase family. Apoptosis induction may be one of the potential mechanisms of cantide-medicated inhibition for tumor cell growth. Continuous cantide treatment might shorten the telomere to a size that leads to cell senescence (Figure 6). The treatment with cantide may be a potential strategy for cancer with telomerase activity.
Edited by Zhang JZ and Wang XL
| 1. | Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Shen ZY, Xu LY, Li EM, Cai WJ, Chen MH, Shen J, Zeng Y. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol. 2002;8:357-362. [PubMed] |
| 3. | Granger MP, Wright WE, Shay JW. Telomerase in cancer and aging. Crit Rev Oncol Hematol. 2002;41:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2004] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 5. | Kim NW. Clinical implications of telomerase in cancer. Eur J Cancer. 1997;33:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Helder MN, Jong S, Vries EG, Zee AG. Telomerase targeting in cancer treatment: new developments. Drug Resist Updat. 1999;2:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Stewart SA, Weinberg RA. Telomerase and human tumorigenesis. Semin Cancer Biol. 2000;10:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1601] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 9. | Wang X, Zhang Z, Xu Y, Chen S, Xiong W. [Inhibition of telomerase activity and induction of apoptosis in lung cancer cell by human telomerase reverse transcriptase gene antisense oligodeoxynucleotide]. Zhonghua Nei Ke Za Zhi. 2002;41:175-178. [PubMed] |
| 10. | Adah SA, Bayly SF, Cramer H, Silverman RH, Torrence PF. Chemistry and biochemistry of 2',5'-oligoadenylate-based antisense strategy. Curr Med Chem. 2001;8:1189-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Mukai S, Kondo Y, Koga S, Komata T, Barna BP, Kondo S. 2-5A antisense telomerase RNA therapy for intracranial malignant gliomas. Cancer Res. 2000;60:4461-4467. [PubMed] |
| 12. | Koga S, Kondo Y, Komata T, Kondo S. Treatment of bladder cancer cells in vitro and in vivo with 2-5A antisense telomerase RNA. Gene Ther. 2001;8:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168-4172. [PubMed] |
| 14. | Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J Neurochem. 2000;75:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Wang SQ, Lin L, Chen ZD, Lin RX, Chen SH, Guan W, Wang XH. Effect of antisense oligonucleotides targeting telomerase cata-lytic subunit on tumor cell proliferation in vitro. Chinese Sci Bulletin. 2002;47:993-997. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Green DW, Roh H, Pippin J, Drebin JA. Antisense oligonucleotides: an evolving technology for the modulation of gene expression in human disease. J Am Coll Surg. 2000;191:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Counter CM, Meyerson M, Eaton EN, Ellisen LW, Caddle SD, Haber DA, Weinberg RA. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 308] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Chang JT, Chen YL, Yang HT, Chen CY, Cheng AJ. Differential regulation of telomerase activity by six telomerase subunits. Eur J Biochem. 2002;269:3442-3450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Takahashi A. Caspase: executioner and undertaker of apoptosis. Int J Hematol. 1999;70:226-232. [PubMed] |
| 20. | Hahn WC, Meyerson M. Telomerase activation, cellular immortalization and cancer. Ann Med. 2001;33:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Yang Y, Chen Y, Zhang C, Huang H, Weissman SM. Nucleolar localization of hTERT protein is associated with telomerase function. Exp Cell Res. 2002;277:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Klingelhutz AJ. The roles of telomeres and telomerase in cellular immortalization and the development of cancer. Anticancer Res. 1999;19:4823-4830. [PubMed] |
| 23. | Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1606] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 24. | White LK, Wright WE, Shay JW. Telomerase inhibitors. Trends Biotechnol. 2001;19:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Kondo Y, Koga S, Komata T, Kondo S. Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telomerase RNA component. Oncogene. 2000;19:2205-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Kumar S. Regulation of caspase activation in apoptosis: implications in pathogenesis and treatment of disease. Clin Exp Pharmacol Physiol. 1999;26:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1305] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 28. | Van de Craen M, Van Loo G, Pype S, Van Criekinge W, Van den brande I, Molemans F, Fiers W, Declercq W, Vandenabeele P. Identification of a new caspase homologue: caspase-14. Cell Death Differ. 1998;5:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Kushner DM, Paranjape JM, Bandyopadhyay B, Cramer H, Leaman DW, Kennedy AW, Silverman RH, Cowell JK. 2-5A antisense directed against telomerase RNA produces apoptosis in ovarian cancer cells. Gynecol Oncol. 2000;76:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Ren JG, Xia HL, Tian YM, Just T, Cai GP, Dai YR. Expression of telomerase inhibits hydroxyl radical-induced apoptosis in normal telomerase negative human lung fibroblasts. FEBS Lett. 2001;488:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |