INTRODUCTION
In hepatoma gene therapy, the technical difficulty is that exogenous gene expression level is too low to achieve therapeutic effecets on in target cells[1]. Studies have been focused on improving gene therapeutic vector, restraining the lysosome activity in target cells, increasing exogenous gene outputs from endosomal vesicles, and modifying their transcription elements[2-7]. Whether there are some relationships between molecular conformation of gene drugs and exogenous gene expression level is not clear. Asialoorosomucoid (ASOR), the specific ligand of asialoglycoprotein receptor on the surface of hepatocytes, can be combined covalently with poly-l-lysine (PLL) to form a soluble DNA transfection vector[8-10]. Human interleukin 12 (hIL-12), also named as cytotoxin lymphocyte maturation factor or natural killer cell stimulatory factor, plays a key regulatory role in humoral immune reactions and explicits bioactivities of antivirus, antitumor and antimetastasis[11-14]. After constructing a hIL12 (human interleukin 12) double-subunit co-expressing plasmid and single-subunit gene expression plasmids, we linked them with ASOR-PLL through electrostatic interactions to form two targeting gene drugs-ASOR-PLL-DNA complexes, then examined their molecular shapes and sizes at various concentration of adjuvant under transmission electron microscope. We compared exogenous gene expression levels to select the best molecular conformation of the complexes 48 hours after transfecting them into HepG2 (ASGr+) cells, and found the means to improve the expression efficiency in target cells on the basis of molecular conformations of gene drugs.
MATERIALS AND METHODS
Materials
Eukaryotic expressing plasmids [pcDNA3.1 (+/-)] and host bacteria E. coli JM109 were obtained from the National Laboratory of Medical Genetics of China. Plasmids purification kit 2500 was purchased from Qiagen (Chatsworth CA). All the restriction endonuclease enzymes were purchased from New England Biolabs (Beverly MA). Minimum essential medium (MEM), Trizol and lipofectamine were purchased from GibcoBRL (Grand Island NY). RT-PCR kit was purchased from Promega (Madison WI). hIL-12 (P70) ELISA kit was purchased from Pharmingen (San Diego CA). Hepatoma cells (HepG2) were obtained from China Center of Type Culture Collection (CCTCC). Newborn bovine serum was purchased from Sijiqing (Hangzhou, China). Chloroquine was a gift from Shanghai Zhong-Xi Pharmaceutical Company. All other reagents were of analytical grade from various companies in China. ASOR-PLL was prepared by ourselves.
Methods
Construction of double-subunit co-expressing plasmid of human interleukin12 The full length cDNAs of P40 and P35 subunits were amplified from human embryonic kidney by RT-PCR based on sequences deposited in Genbank (P40 accession number AF180563, P35 accession number AF180562), and cloned into pcDNA3.1 (+/-) to obtain P (+)/P40 and P (-)/P35 plasmids. The segments-P35-polyA and -cmv-P40-were amplified from P (-)/P35 and P (+)/P40 by using the following primers IL40F, IL40R, IL35F, IL35R. They were then ligated and cloned into pcDNA3.1 (+) to obtain double-subunit co-expressing plasmid P (+)/IL-12 (Figure 1).
Figure 1 Primers for amplifying human interleukin 12.
Observation under transmission electron microscope After the three plasmids were mixed and incubated with ASOR-PLL at various molecular weight ratios for 40 minutes at room temperature, the mixture was electrophoresed in a 0.8% agarose gel retardation system to determine its optimal ratio. ASOR-PLL-P (+)/P40, ASOR-PLL-P (-)/P35 and ASOR-PLL-P (+)/IL-12 formed in optimal ratios were the two targeting gene drugs. These two drugs (plasmid DNA 0.9 μg) were formed at various concentrations of adjuvant (0, 0.1 M, 0.2 M, 0.3 M, 0.4 M, 1.5 M), then their A260 values were measurated and examined under a transmission electron microscope after contrast staining by 2% uranyl acetate (bar = 100 nm).
Cell culture and transfection HepG2 cells were cultured to 2 × 107 in minimum essential media plus 15% fetal bovine serum (37 °C, 5% CO2), and then subcultured in 12-well plates at 1:3 ratio. After the cells were cultured to 60%-70% confluence, the 6 molecule conformations of ASOR-PLL-P (+)/IL-12 and the combined ASOR-PLL-P (+)/P40 and ASOR-PLL-P (-)/P35 were transfected into the cells using liposome transfection as positive control. There were three wells in each group and the total transfected plasmid DNA was 4.5 μg for each well. During transfection, chloroquine was used (the final concentration was 100 μM) to restrain lysosome activity[15,16].
Detection of hIL12 expression Total RNA of transfected HepG2 was extracted by using Trizol 48 hours after transfection. mRNA expression of P40 and P35 was determined with semi-quantitative RT-PCR using β-actin as an internal control. hIL12 protein in the supernatant was measured using ELISA.
RESULTS
Construction of P (+) P40, P (-) P35 and P (+) IL12 plasmids
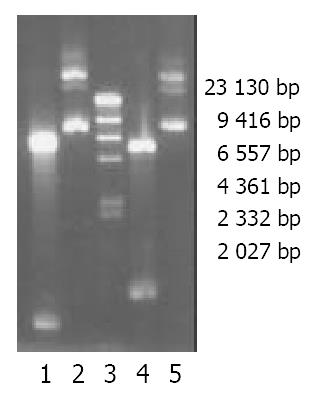
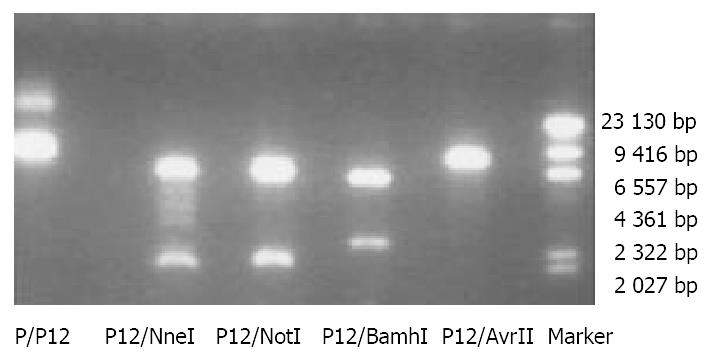
The full length cDNAs of P40 and P35 subunits were amplified from human embryonic kidney and cloned into pcDNA3.1 (+/-). P (+)/P40 plasmids (6.4 kb) could be cut by Kpn I and Xho I to produce two bands: 5.4 kb and 1.007 kb while P (-)/P35 plasmids could be cut Kpn I and Nhe I to produce 5.4 kb and 823 bp bands (Figure 2). The segments of -P35-polyA and -cmv-P40- were ligated and cloned into pcDNA3.1 (+) to get P (+)/IL-12 (8.51 kb). P (+)/IL-12 could be cut by Nhe I and Not I (2.5 kb and 6 kb) or by BamH I (2 kb and 6.5 kb) or linearized by Avr II (8.51 kb) (Figure 3).
Figure 2 Electrophoresis of P (-)/P35, P (+)/P40 and their bands after restrictive endonuclease enzyme digestion in a 0.
8% aga-rose gel. 1. P (-)/P35 plasmid digested by Kpn I and Nhe I. 2. P (-)/P35 plasmid. 3. Marker (λDNA/Hind III). 4. P (+)/P40 plasmid. 5. of P (+)/P40 plasmid cut by Kpn I and Xho I.
Figure 3 Electrophoresis of recombinant expression plasmid P (+)/IL-12 and the bands after restriction enzyme digestion in a 0.
8% agarose gel (Marker: λDNA/Hind III).
Molecular conformations of targeting gene drugs at various concentrations of adjuvant under transmission electron microscope
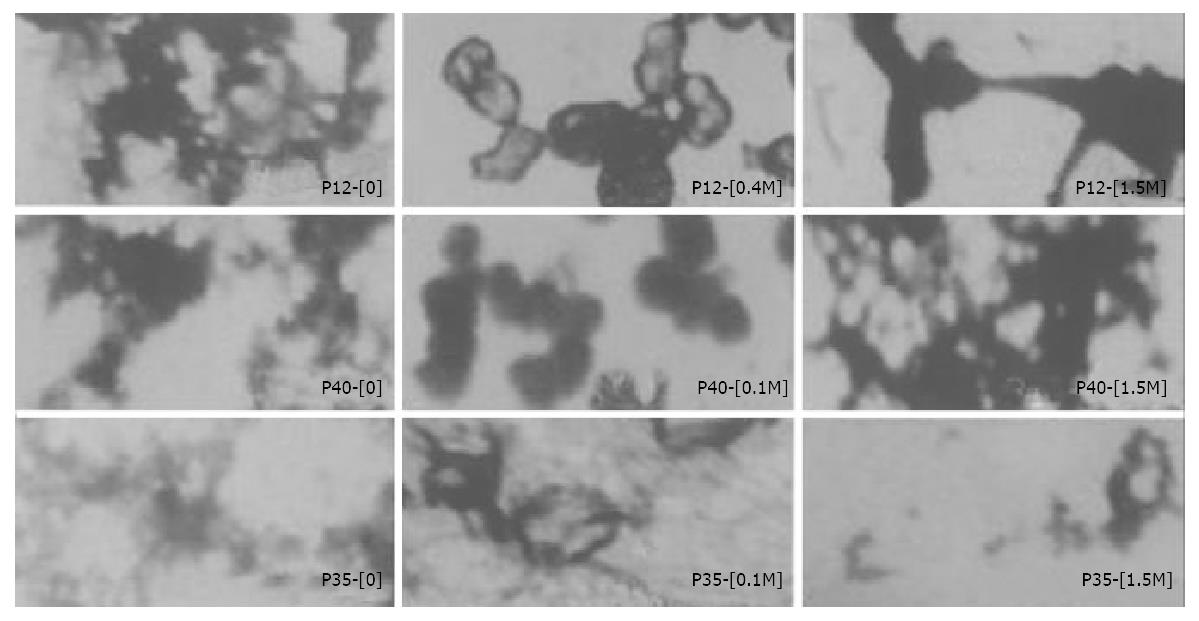
The optimal ratios of ASOR-PLL: DNA were as follow: ASOR-PLL:P (+)/IL-12 = 4:1, ASOR-PLL:P (+)/P40[P (-)/P35] = 2:1. ASOR-PLL-DNA complexes which formed in 0, 0.1 M, 0.2 M, 0.3 M, 0.4 M, 1.5 M adjuvant were examined under a transmission electron microscope. The molecular conformations of gene drugs showed divarication-like (ψ-DNA) without adjuvant or in 0.3 M adjuvant, the drug molecules were condensed to granules or “doughnut”-like structures with diameters of 25 nm-300 nm in 0.1 M, 0.2 M and 0.4 M adjuvant. Their conformations varied greatly in 1.5 M adjuvant. The structure of ASOR-PLL-P (+)/IL-12 was rod-like or granule-like. The structures of ASOR-PLL-P (+)/P40 and ASOR-PLL-P (-)/P35 were both divarication-like (Figure 4).
Figure 4 Differently structural features of targeting gene drugs (ASOR-PLL-DNA complexes) at various concentrations of adjuvant under transmission electron microscope.
(The bar equals 100 nm, amplified 40000 times).
Detection of hIL-12 expression 48 hours after drugs with different molecular conformations were transfected into HepG2 cells
Total RNA was extracted and semi-quantitative RT-PCR was performed using β-actin as an internal control. hIL12 mRNA levels were increased in both liposome-transfected and ASOR-PLL transfected groups. mRNA levels in ASOR-PLL groups varied with different concentrations of adjuvant (data not shown).
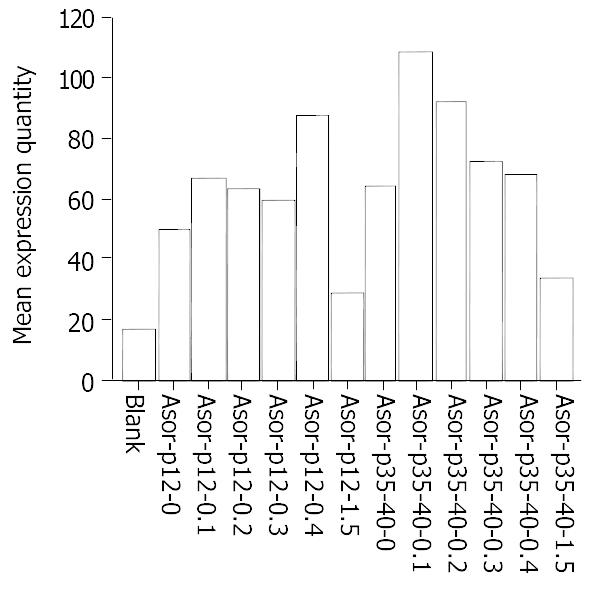
ELISA results showed that the highest expression level of hIL12 was achieved in cells transfected with ASOR-PLL-P (+)/IL-12 in 0.4 M adjuvant, while the same level of expression was achieved when ASOR-PLL-P (+)/P40 and ASOR-PLL-P (-)/P35 were co-transfected into HepG2 cells in 0.1 M adjuvant. The expression levels in both groups went down to the lowest points in 1.5 M adjuvant. The expression levels in ASOR-PLL-P (+)/P40 and ASOR-PLL-P (-)/P35 co-transfected group were higher than those in ASOR-PLL-P (+)/IL-12 group at the same concentrations (Figure 5).
Figure 5 ELISA results of hIL-12 expressed in cell supernatant 48 hours after targeting gene drugs at various adjuvant concentrations were transfected into HepG2.
DISCUSSION
Gene transfection system mediated by receptor-ligand binding, which possesses some advantages for gene therapy, including transferring foreign genes to target cells specifically, being safer without mutation and infection caused by viral vectors, expressing functional proteins without integrating foreign genes into genomic DNA, and unlimited foreign gene size theoretically[17-19], is a potential gene therapy vector which can substitute viral vectors. ASOR is the ligand of ASG receptor (ASGr) which locates specifically on the surface of hepatocytes, and can bind to ASGr specifically[20,21]. Through the positively charged linking molecule PLL which binds to negatively charged DNA molecules through electrostatic interactions, ASOR can transfer foreign DNA molecules into hepatocytes or some hepatoma cells (ASGr+) and express the functional proteins. In 1987, Wu GY and Wu CH firstly transfected reporter gene chloramphenicol acetyltransfectase (CAT) to hepatoma cells (HepG2) by using ASOR-PLL[8]. Ever since, ASOR-PLL has been applied to various therapeutic genes targeting for hepatocytes or some hepatoma cells in vivo or in vitro[17,22,23]. However, the low expression efficiency of exogenous genes restricts its application to clinic use.
hIL-12 is a double-subunit cytokine discovered in recent years[24], which can promote Th0 cells to differentiate Th1 cells to enhance cell immunity[25]. It can also activate T cells and NK cells to proliferate and secrete IFN-γ to improve humoral immunity[26,27]. Therefore, it plays a role in antivirus, antiprimary tumors and antimetastasis[11-14]. Till now ASOR-PLL has not been used in transferring human interleukin 12 gene to HepG2. We constructed hIL12 double-subunit co-expressing plasmid P (+)/IL12 and single-subunit gene expressing plasmids P (+)/P40 and P (-)/P35, and bound them to ASOR-PLL at various adjuvant concentrations and measured A260 values of the mixtures (data not shown). ASOR-PLL-P (+)/IL-12 showed the lowest A260 value in 0.4 M adjuvant which indicated the least dissociated DNA molecules and the highest hIL-12 expression level, while ASOR-PLL-P (+)/P40 and ASOR-PLL-P (-)/P35 showed the same result in 0.1 M adjuvant. Though ASOR-PLL-DNA complexes showed lower A260 values in 1.5 M adjuvant, most of the HepG2 cells could not stand such a high concentration of adjuvant and died 4 hours after they were transfected, which explained why the expression level of hIL-12 was low. These results indicate that foreign genes (hIL12) can be transferred into target cells (HepG2) through ligand (ASOR) binding to receptors (ASGr), and then endocytosed by cells as reported by Huckett et al[28]. The less dissociated DNA molecules existed in the mixture, more ASOR-PLL-DNA complexes were formed, the higher efficiency of expressing hIL12 was achieved.
Pearales et al[17] thought that ligand-DNA complex molecules in a receptor-mediated DNA transfection system must have a relatively high DNA concentration and certain molecule conformations to assist DNA to be transfected into target cells and express functional proteins. To achieve this goal, DNA molecules need to be condensed to a smaller size, and the conformation of aggregated multiple ligand-DNA molecules (such as ψ-DNA) inhibited gene expression. In our studies, shapes of the molecules under transmission electron microscope showed that ASOR-PLL-P (+)/IL-12 molecules were circle-like structures (“doughnut”) with diameters of 50-100 nm in 0.4 M adjuvant, which produced the highest hIL-12 expression. ASOR-PLL-P (+)/P40 molecules were granular structures with diameters of 40-125 nm in 0.1 M adjuvant and ASOR-PLL-P (-)/P35 molecules were circle-like structures with diameters of 75-150 nm at the same concentration of adjuvant, both of them also achieved the highest hIL12 expression. Although ASOR-PLL-P (+)/P40 molecules formed granular structures with diameters of 25-75 nm in 0.2 M adjuvant, ASOR-PLL-P (-)/P35 molecules were concentrated to form lumps with diameters of 100-200 nm at this concentration of adjuvant. Since ASOR-PLL-P (+)/P40 and ASOR-PLL-P (-)/P35 needed to be co-transfected to express functional hIL-12, there were more factors affecting hIL-12 expression in this situation. Meanwhile, the size and the GC/AT ratio of foreign DNA molecules also affected exogenous gene expression[17]. In our studies, the smaller molecular diameters of ASOR-PLL-DNA complexes (25-150 nm), the more condensed DNA molecules and the tighter combination of DNA molecules and ASOR-PLL could lead to higher hIL-12 expression.
In general, single-subunit gene plasmids [P (+)/P40 and P (-)/P35] co-expression mediated by ASOR-PLL could induce higher hIL12 expression than that of double-subunit genes co-expressing plasmid transfection. The possible reasons might be the following: (1) There was a certain amount of ASGr on the surface of target cells, when the same amount of DNA was used to transfect cells, ASOR-PLL-P (+)/P40 and ASOR-PLL-P (-)/P35 were co-transfected at 1:1 ratio, and ASOR could bind to ASGr more thoroughly with a relatively larger amount of ASOR, compared with ASOR-PLL-P (+)/IL-12 transfection. (2) Since the P40 and P35 subunits cDNAs were cloned in tandem into the polycloning site of pcDNA3.1 (+) in which P40 cDNA located directly next to P35 cDNA, they might interfere reciprocally during transcription, and could not produce P40 and P35 subunits proportionally. However, P40 and P35 subunits must combine at 1:1 ratio to form functional hIL-12[12,25].
Another study[29], used atomic force microscope to examine DNA molecules binding to PL, ASOR-PLL and orosomucoid, and found that the optimal conformations of DNA complex molecules for foreign genes expression were solenoid-like or rod-like in diameters of 300 nm-400 nm. The condition to obtain these conformations was that ASOR was combined covalently to 10 kDa PL (Lys: nt ≥ 5:1), and linked to DNA molecules. In our studies, PLL was a mixture of 26 kDa, 10 kDa and 4 kDa PLL. The ratio of 10 kDa PL: nucleotide was less than 5:1 in our targeting gene drugs, so hIL-12 expression in ASOR-PLL transfection group was lower than that in liposome transfection group (data not shown).
In summary, the gene transfection system mediated by receptor-ligand binding is a prospective gene therapy strategy, and the expression level of foreign genes has a relationship with the amount of binding DNA, conformation of the complex and characteristics of the linking molecules PLL. Since hIL-12 consists of two subunits (P40 and P35), more factors are involved in hIL-12 expression, including the way P40 and P35 genes enter into the target cells, conformations of the two ASOR-PLL-DNA complexes and interactions of the two complexes. Our studies may contribute to improving hIL-12 expression and applying hIL-12 to clinical hepatoma gene therapy research.