Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.1897
Revised: May 23, 2003
Accepted: June 2, 2003
Published online: September 15, 2003
AIM: To investigate the special Fourier transform infrared spectroscopy (FT-IR) spectra in normal and cancerous tissues of esophagus.
METHODS: Twenty-seven pairs of normal and cancerous tissues of esophagus were studied by using FT-IR and the special spectra characteristics were analyzed in different tissues.
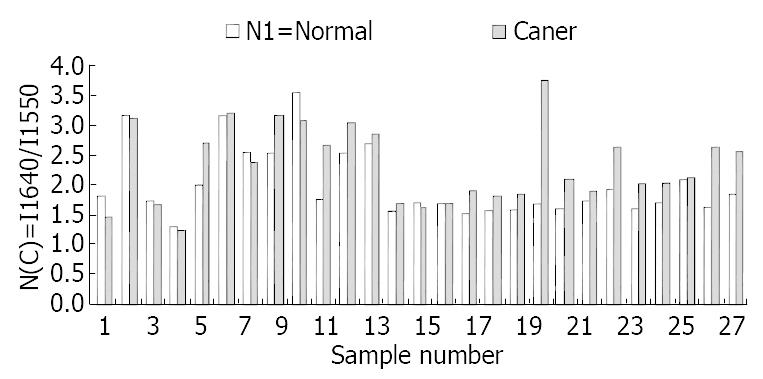
RESULTS: Different spectra were found in normal and cancerous tissues. The peak at 1550/cm was weak and wide in cancerous tissues but strong and high in normal tissues.The ratio of I 1647/I 1550 was 2.0 in normal tissues and 2.36 in cancerous tissues (P < 0.05). The ratio of I 1550/I 1080 was 4.5 in normal tissues and 3.4 in cancerous tissues (P < 0.01). The peak at 1453/cm was higher than at 1402/cm in normal tissue and lower than at 1402/cm in cancerous tissues.
CONCLUSION: The results indicate that FTIR may be used in clinical diagnosis.
- Citation: Wang JS, Shi JS, Xu YZ, Duan XY, Zhang L, Wang J, Yang LM, Weng SF, Wu JG. FT-IR spectroscopic analysis of normal and cancerous tissues of esophagus. World J Gastroenterol 2003; 9(9): 1897-1899
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/1897.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.1897
Esophageal carcinoma is still one of the malignant tumors that challenges clinical oncologists. In China, there are about 1300000 new patients diagnosed as esophageal carcinoma each year[1]. At present, the best way to diagnose the disease is fiber endoscopy, which can not only look steadily at the lesion to get information of the size, location, ect, but also obtain the tissues of suspected portions for pathological examinations. Although endoscopy has been widely used in diagnosis and differential diagnosis of esophageal diseases, about 20 percent of esophageal cancer in early stage can not be detected and diagnosed by this technology because it is limited by the experience of the operator and affected by the atypical clinical manifestations of the disease[2].
Thus, it is the very goal to develop an accurate, quick, convenient, and inexpensive method for detecting early cancer of esophagus at molecular level. With the development of vibrational spectroscopic technologies, their application in cancer research has been reported. Among these technologies, Fourier transform infrared spectroscopy (FT-IR) has been extensively employed in the fields of biology and chemical engineering to get information about the structural and chemical/physical properties at molecular level. Recently, some researches about the application of FT-IR in cancer have been reported[3-8]. In fact, the results of our previous research have already shown its advantages in clinical and scientific applications[9-14]. But the study on the spectra of esophageal tissues is still preliminary. In the present work, we examined the cancerous and normal tissues of esophagus with FT-IR in order to put some light on the combination of FT-IR and endoscopy.
Twenty-seven fresh tissues of esophageal cancer were obtained from the First Hospital of Xi’an Jiaotong University. The tissues were washed with normal saline solution and sampled immediately after removed during the operation. Two pieces of the tissues about 1 cm in diameter were taken, one was cut off from the center of the lesion and the other was from the distant edge of the removed tissues. Each tissue was divided into two equal parts, one was frozen with liquid nitrogen and the other was fixed with 10% formalin, embedded in paraffin and stained with hematoxylin and eosin for pathological examination. All the 27 patients were diagnosed before operation as squamous cell carcinoma with endoscopic biopsy. There were 15 males and 12 females, aged 41 to 71 years old, and averaged 58 years.
The spectra were recorded on a Nicolet Magna 750 FT-IR spectrometer with a mercury cadmium telluride (MCT) detector. No special sample preparation was needed in the experiment. The sample was defrosted at room temperature, and the measurements were carried out by putting the mucosal surface and cancer side (the section side of cancer tissue) of tissue on the attenuated total reflectance (ATR) probe. A total of 64 scans were recorded at a resolution of 4/cm at the region of 900 to 4000/cm. The samples were frozen again for reexamination after FT-IR scanning.
Significant differences were found between normal and malignant esophageal tissues in the FT-IR spectra except one sample.
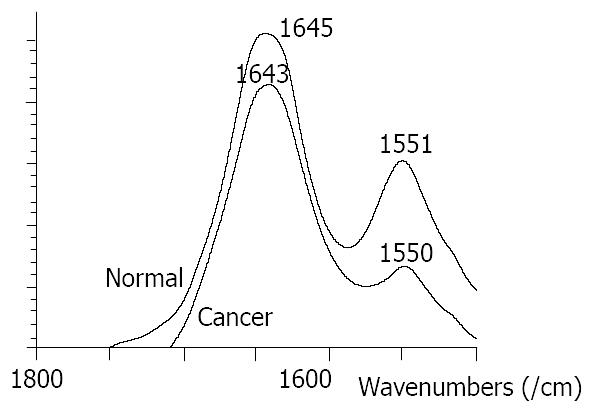
At the range of 1800-1500/cm, the amide band II at about 1550/cm was weak and broad in malignant tissues, and was strong and sharp in normal tissues (Figure 1). The relative intensity of I 1647/I 1550 of malignant tissues was higher than that of normal tissues (Figure 2). The ratio of I 1647/I 1550 was 2.0 in malignant tissues and 2.36 in normal tissues, with a statistically significant difference (P < 0.05). The wave number of amide band I in normal tissues was about 1-6/cm higher than that in malignant tissues, without statistically significant difference (P > 0.05).
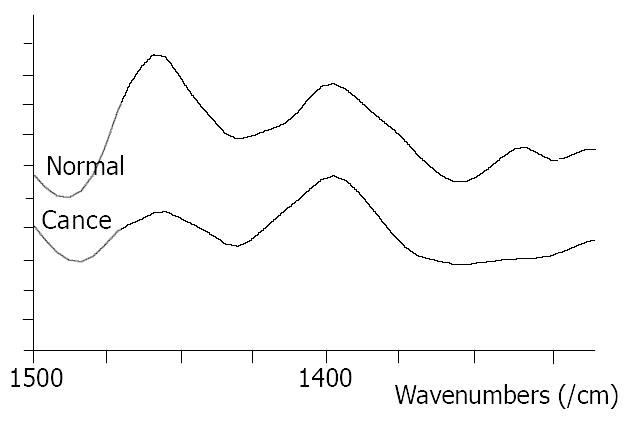
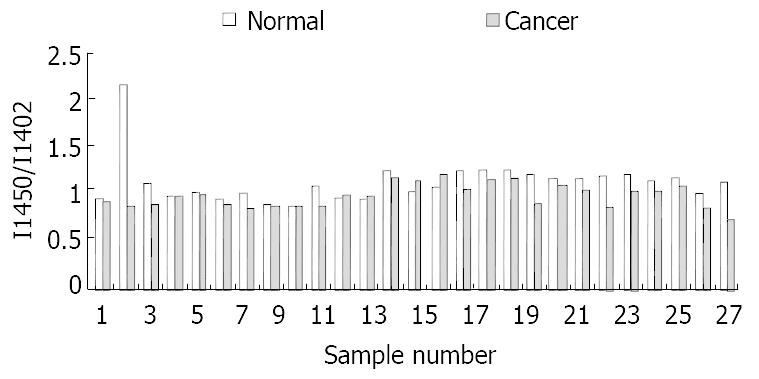
At the range of 1500-1400/cm, two typical bands at about 1453/cm and 1402/cm could be seen in all the studied samples. The intensity of the band at 1453/cm in normal tissues was higher than that in malignant tissues (Figure 3). The relative intensity of I 1454/I 1400 is shown in Figure 4.
The band at 1080/cm was higher and stronger in malignant tissues than that in normal tissues. The ratio of I 1080/I 1540 was higher in malignant tissues. In normal tissues, a peak at 1745/cm was seen in 6 cases, but none was seen in malignant tissues. At the range of 2800-3000/cm, three peaks (2965/cm, 2926/cm, and 2853/cm) were seen in 25 of 27 normal tissues and only in 5 of both normal and malignant tissues. For these 5 cases, the ratio of I 1226/I 3380 was higher in normal tissues. For a pair of samples, in which the same spectra were found by FT-IR, the regular pathological examination showed that the two samples were normal tissues of esophagus.
The pathogenesis and development of esophageal carcinoma are a multi-step process that is controlled by many genes and affected by many factors[15-24]. The cooperation of oncogene’s activation and anti-oncogene’s inactivation causes the relative changes of contents and structures of protein, nuclear acid, sugar and fat in cells[25-31]. FT-IR could provide information about molecular structure, which makes it possible to reflect the changes of protein, nuclear acid, sugar and fat in cells and the structure changes of space array of the molecule, thus the diagnosis of cancer cells could be made at the molecular level[32-34].
Significant differences between normal and malignant esophageal tissues were seen in FT-IR spectra in this study. They might be caused by the changes of content and space array of protein, nuclear acid, sugar and fat in cells. For the bands of protein, amide bands I and II, the amide band II at about 1550/cm was weak and broad in malignant tissues and was strong and sharp in normal tissues. The relative intensity of I 1647/I 1550 of malignant tissues was higher than that of normal tissues. At the range of 1500-1400/cm, the relative intensity of I 1454/I 1402 was higher in normal tissues than that in malignant tissues.
The peak at 1745/cm was caused by the stretching vibration of carbonyl, the peaks at 2852/cm and 2930/cm were absorption spectra of the symmetry and asymmetry stretching vibrations of methylene, the peaks at 2873/cm and 2958/cm were absorption spectra of the symmetry and asymmetry stretching vibrations of methyl. Because triglyceride contains lots of methyl, methylene and carbonyl, so the differences of FT-IR spectra in normal and malignant tissues at those area were caused by the different contents of lipid substance in the tissues. The decreased lipid substance in malignant tissues might be related to the following factors. Firstly, the fast growth of cancer needs more nutrition and energy, therefore it might utilize lipid to supply nutrition and energy, which cause the lipid substance not to be accumulated in cancer cells and tissues. The second reason is that normal cell and tissues, including fat cells, are excluded mechanically by proliferating malignant tissues of the tumor.
One of the most important characteristics of cancer cells is the increased DNA content in cells caused by the endless replication of DNA, which is presented with enlarged and dark stained nuclei under light microscopy. The peak at 1080/cm was caused by symmetry stretch ing vib ration s of phospholipids. In this study, we found the peak at 1080/cm was stronger and higher in malignant tissues than that in normal tissues and the ratio of I 1080/I 1540 was higher in malignant tissues, indicating that DNA content in malignant tissues is significantly higher than that in normal tissues.
Edited by Zhang JZ and Wang XL
| 1. | Shiozaki H, Tahara H, Kobayashi K, Yano H, Tamura S, Imamoto H, Yano T, Oku K, Miyata M, Nishiyama K. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990;66:2068-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Tachimori Y, Kato H. Diagnosis and surgery of esophageal cancer. Crit Rev Oncol Hematol. 1998;28:57-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Yamada T, Miyoshi N, Ogawa T, Akao K, Fukuda M, Ogasawara T, Kitagawa Y, Sano K. Observation of molecular changes of a necrotic tissue from a murine carcinoma by Fourier-transform infrared microspectroscopy. Clin Cancer Res. 2002;8:2010-2014. [PubMed] |
| 4. | Argov S, Ramesh J, Salman A, Sinelnikov I, Goldstein J, Guterman H, Mordechai S. Diagnostic potential of Fourier-transform infrared microspectroscopy and advanced computational methods in colon cancer patients. J Biomed Opt. 2002;7:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Yano K, Ohoshima S, Gotou Y, Kumaido K, Moriguchi T, Katayama H. Direct measurement of human lung cancerous and noncancerous tissues by fourier transform infrared microscopy: can an infrared microscope be used as a clinical tool. Anal Biochem. 2000;287:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Romeo MJ, Wood BR, Quinn MA, McNaughton D. Removal of blood components from cervical smears: implications for cancer diagnosis using FTIR spectroscopy. Biopolymers. 2003;72:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Wong PT, Senterman MK, Jackli P, Wong RK, Salib S, Campbell CE, Feigel R, Faught W, Fung Kee Fung M. Detailed account of confounding factors in interpretation of FTIR spectra of exfoliated cervical cells. Biopolymers. 2002;67:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Ramesh J, Kapelushnik J, Mordehai J, Moser A, Huleihel M, Erukhimovitch V, Levi C, Mordechai S. Novel methodology for the follow-up of acute lymphoblastic leukemia using FTIR microspectroscopy. J Biochem Biophys Methods. 2002;51:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Peng Q, Xu Y, Li W, Wu J, Zhou X. [FTIR study on the normal and tumor gastrointestinal tissues]. Guangpuxue Yu Guangpufenxi. 1998;18:528-531. [PubMed] |
| 10. | Xu YZ, Soloway RD, Lin XF, Zhi X, Weng SF, Wu QG, Shi JS, Sun WX, Zhang TX, Wu JG. Fourier transform infra-red (FT-IR) mid-IR spectroscopy separates normal and malignant tissue from the colon and stomach. Gastroenterology. 2000;118:6438. [DOI] [Full Text] |
| 11. | Wu JG, Xu YZ, Sun CW, Soloway RD, Xu DF, Wu QG, Sun KH, Weng SF, Xu GX. Distinguishing malignant from normal oral tissues using FTIR fiber-optic techniques. Biopolymers. 2001;62:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Ma J, Wang JS, Soloway RD, Xu YZ, Wang F, Zhang L, Yang LM, Shi JS, Wu JG. Separation of normal from diseased hepatocytes using Fourier transform infrared (FT-IR) spectroscopy: A basis for histologic characterization and quantitation.. Gastroenterology. 2002;122:1467. |
| 13. | Weng SF, Ling XF, Song YY, Xu YZ, Li WH, Zhang X, Yang L, Sun W, Zhou X, Wu J. FTIR fiber optics and FT-Raman spectroscopic studies for the diagnosis of cancer. Am Clin Lab. 2000;19:20. [PubMed] |
| 14. | Wang JS, Shi JS, Xu YZ, Weng SF, Wu JG. Preliminary FT-IR study on the normal, inflammatory and malignant tissues of gallbladder. Zhonghu Gandan Waike Zazhi. 2002;8:196. |
| 15. | Zhou J, Zhao LQ, Xiong MM, Wang XQ, Yang GR, Qiu ZL, Wu M, Liu ZH. Gene expression profiles at different stages of human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9:9-15. [PubMed] |
| 16. | Xu M, Jin YL, Fu J, Huang H, Chen SZ, Qu P, Tian HM, Liu ZY, Zhang W. The abnormal expression of retinoic acid receptor-beta, p 53 and Ki67 protein in normal, premalignant and malignant esophageal tissues. World J Gastroenterol. 2002;8:200-202. [PubMed] |
| 17. | Wang AH, Sun CS, Li LS, Huang JY, Chen QS. Relationship of tobacco smoking CYP1A1 GSTM1 gene polymorphism and esophageal cancer in Xi'an. World J Gastroenterol. 2002;8:49-53. [PubMed] |
| 18. | Chen H, Wang LD, Guo M, Gao SG, Guo HQ, Fan ZM, Li JL. Alterations of p53 and PCNA in cancer and adjacent tissues from concurrent carcinomas of the esophagus and gastric cardia in the same patient in Linzhou, a high incidence area for esophageal cancer in northern China. World J Gastroenterol. 2003;9:16-21. [PubMed] |
| 19. | Li J, Feng CW, Zhao ZG, Zhou Q, Wang LD. A preliminary study on ras protein expression in human esophageal cancer and precancerous lesions. World J Gastroenterol. 2000;6:278-280. [PubMed] |
| 20. | Li X, Lu JY, Zhao LQ, Wang XQ, Liu GL, Liu Z, Zhou CN, Wu M, Liu ZH. Overexpression of ETS2 in human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9:205-208. [PubMed] |
| 21. | Millar CB, Guy J, Sansom OJ, Selfridge J, MacDougall E, Hendrich B, Keightley PD, Bishop SM, Clarke AR, Bird A. Enhanced CpG mutability and tumorigenesis in MBD4-deficient mice. Science. 2002;297:403-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848-854. [PubMed] |
| 23. | Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN, Wu M. Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966-970. [PubMed] |
| 24. | Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 588] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 25. | Ginn-Pease ME, Eng C. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res. 2003;63:282-286. [PubMed] |
| 26. | Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, Behm FG, Pui CH, Downing JR, Gilliland DG. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 822] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 27. | Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Rincón-Arano H, Rosales R, Mora N, Rodriguez-Castañeda A, Rosales C. R-Ras promotes tumor growth of cervical epithelial cells. Cancer. 2003;97:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196-206. [PubMed] |
| 30. | Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Wu BW, Wu Y, Wang JL, Lin JS, Yuan SY, Li A, Cui WR. Study on the mechanism of epidermal growth factor-induced proliferation of hepatoma cells. World J Gastroenterol. 2003;9:271-275. [PubMed] |
| 32. | Dovbeshko GI, Chegel VI, Gridina NY, Repnytska OP, Shirshov YM, Tryndiak VP, Todor IM, Solyanik GI. Surface enhanced IR absorption of nucleic acids from tumor cells: FTIR reflectance study. Biopolymers. 2002;67:470-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Lasch P, Pacifico A, Diem M. Spatially resolved IR microspectroscopy of single cells. Biopolymers. 2002;67:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Neviliappan S, Fang Kan L, Tiang Lee Walter T, Arulkumaran S, Wong PT. Infrared spectral features of exfoliated cervical cells, cervical adenocarcinoma tissue, and an adenocarcinoma cell line (SiSo). Gynecol Oncol. 2002;85:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |