Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1381
Revised: October 24, 2002
Accepted: November 4, 2002
Published online: June 15, 2003
We reported a case of non-Hodgkin's lymphoma where liver involvement was the predominant clinical manifestation. A 27-year old man presented wiht markedly elevated serum aspartate aminotrasferase, alanine aminotransferase and lactate dehydrogenase, reduced prothrombin activity, thrombocytopenic purpura and hepato-splenomegaly without adenopathy. Viral, toxic, autoimmune and metabolic liver diseases were excluded. Bone marrow biopsy showed an intracapillary infiltration of T-lymphocytes with no evidence of lipid storage disease. Because of a progressive spleen enlargement, splenectomy was performed. Histological examination showed lymphomatous intrasinuses invasion of the spleen. Immunohistochemical investigation revealed the T phenotype of the neoplastic cells: CD45+, CD45RO+, CD3+, CD4-, CD8-, TIA1-. About 50% of the lymphoid cells expressed CD56 antigen. The diagnosis of hepatosplenic T cell lymphoma was done. The patient was treated with chemotherapy, which induced a complete remission. Eighteen months later, he had a first relapse with increased aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, thrombocytopenic purpura and blast in the peripheral blood. In spite of autologous bone marrow transplantation, he died twenty months after the diagnosis. Even in the absence of a mass lesion or lymphoadenopathy, hepatosplenic T-cell lymphoma should be considered in the differential diagnosis of a patient whose clinical course is atypical for acute hepatic dysfunction.
- Citation: Perfetto F, Tarquini R, Mancuso F, Lollo SD, Tozzini S, Bellesi G, Laffi G. Hepato-splenic lymphoma: a rare entity mimicking acute hepatitis: A case report. World J Gastroenterol 2003; 9(6): 1381-1384
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1381
Liver and splenic involvement is commonly seen during a malignant lymphoma, but rarely occurs as a prominent clinical feature at diagnosis. Hepatosplenic lymphoma is a rare and poorly recognized entity[1] characterized by neoplastic proliferation of T-cell bearing a γδ or, more rarely, αβ clonal rearrangement of the receptor (TCR). This lymphoma shows a specific morphological pattern characterized by a preferential hepatic sinusoids and splenic red pulp involvement without lymphoadenopathy and discrete or absent bone marrow involvement. Moreover the diagnosis is complicated in several cases by the presence of misleading symptoms. We reported the case of a young man without palpable lymphoadenopathy and clinical and laboratory presentations resembling an acute liver disease.
A 27-year old caucasian man was admitted in an Internal Medicine unit in February 1999 because of fever (38 °C), malaise, anorexia, and erythematous macules and papules on the face, trunk and arms. The patient referred similar but transient cutaneous lesions in the previous two-months. Laboratory investigations yielded the following results: aspartate aminotransferase (AST) 240 IU/L (normal range: 0-37 IU/L), alanine aminotransferase (ALT) 520 IU/L (normal range: 0-40 IU/L), γ-glutamyl-transpeptidase (γ-GT) 46 IU/L (normal range: 11-43 IU/L), alkaline phosphatase (ALP) 298 IU/L (normal range: 60-270 IU/L) and platelets 120 × 109/L (normal range: 130-400 × 109/L). Serological tests for Ebstein Barr virus (EBV) showed a positivity for nuclear antigen (IgG 248 ACU/ml) (normal value: < 56 ACU/ml) whereas serology for hepatitis A (HAV), hepatitis B (HBV) and hepatitis C (HCV) viruses was negative. Physical examination showed a mild hepato-splenomegaly. There was no palpable lymphoadenopathy.
Two weeks later he was transferred to our Internal Medicine unit with the clinical diagnosis of "acute hepatitis with hepato-splenomegaly and recidivant purpuric exanthema". Physical examinations showed an enlarged tender liver (2 cm below the rib border) and splenomegaly (2 cm below the rib border) without peripheral lymphoadenopathy. The cutaneous lesions of the trunk and the face were already present as purpuric palpable papules. Fever was absent. Abdominal ultrasonography revealed a mild hepatomegaly and splenomegaly (major axis of 18 cm), without ascite and abdominal lymphoadenomegaly. Doppler sonography revealed an increased blood flow in the spleen and the portal vein without obstruction of the main hepatic veins. An upper gastrointestinal endoscopy did not reveal esophageal varices. Chest x-rays at the time of admission yielded normal findings. Liver function test showed AST 498 IU/L, ALT 958 IU/L, γ-GT 49 IU/L, ALP 426 IU/L, total bilirubin 1.15 mg/dl (normal range: 0.2-1.0 mg/dl) and lactate dehydrogenase (LDH) 1234 U/L (normal range 190-450 IU/L). Peripheral blood counts showed hemoglobin 16.1 g/dl, hematocrit 45%, leukocytes 4.9 × 109/L with normal differential count and platelets 80 × 109/L. Coagulation indices showed prothrombin activity 53% (normal range: 90%-100%), international normal ratio 1.4, activated partial thromboplastin time 53 sec (normal range: 25-40 sec) and fibrinogen 267 mg/dl (normal range: 200-400 mg/dl). Albumin levels were 4.25 g/dl (normal range: 3.3-5.1 g/dl) and γ-globulin were 1.44 g/dl (normal range: 0.84-1.44 g/dl). Serological tests were negative for HAV, HBV, HCV, cytomegalovirus and human herpes simplex N° 1, 2, and 6. EBV serologic findings were heterophile test (heterophile antibody) negative, viral capsid antigen (VCA) immunoglobulin IgM negative and IgG positive. Serological tests exploring infective agents able to induce an acute hepatitis such as Chlamydia pneumoniae, Bartonella spp, Borrelia Burgdorferi, Toxoplasma Gondii, Coxsackie virus groups A and B (N°1-6) and Echovirus, were negative as well as the serological test for Rickettsie and Parvovirus B19. Results of serology for human immunodeficient virus (HIV) 1 and 2 including cDNA Polymerase Chain Reaction and the screening of the antigen p24 of HIV1 were negative. Serum γ-fetoprotein and carcinoembryonic antigens were within the normal range. To exclude autoimmune disease, serological tests for anti-nuclear, anti-mitochondrial, anti-liver/kidney microsomial (LKM), and anti-smooth muscle (ASM) were performed but the results were negative; cryoglobulins and circulating immune complexes were absent. Serum ceruloplasmine levels, circulating serum copper levels, 24-h urine excretion of copper and α 1-antitrypsin levels were in the normal range and Kayser Fleischer rings were absent on slit-lamp examination. To exclude a thrombocytopenia induced by EDTA (pseudothrombocytopenia) blood was drawn on test tube with sodium citrate as anticoagulant but the result confirmed the presence of a true thrombocytopenia. Anti-platelet antibodies were negative in two consecutive different samples and reticulate platelets were in the normal range. Toxic (including also ingestion of hepatotoxic mushrooms such as Amanita Phalloides) and drugs inducing hepatitis were excluded by a careful and detailed personal and family history. During the following weeks, patient complained increasing fatigue and tenderness in the upper left quadrant and physical examination revealed an enlarged spleen that extended his lower pole 4-cm below the rib border. Liver function tests continued to rise with AST 658 IU/L, ALT 1074 IU/L, γ-GT 72 IU/L, ALP 473 IU/L, and LDH 1365 U/L. Serum β-2 microglobulin levels were 2.4 mg/dl; (normal range: 1.2-2.5 mg/dl). Coagulation indices showed prothrombin activity 51%, international normal ratio 1.4, fibrinogen 146 mg/dl and activated partial thromboplastin time 42.4 sec without serological evidence of circulating anticoagulants such as lupus anticoagulant or anticardiolipin antibodies. A decreased activity of coagulation factor II (57%; normal range: 70%-120%), VII (58%; normal range: 70%-120%) and X (41%; normal range: 70%-120%) were also demonstrated, despite i.v. Vitamin K treatment; factor VIII activity was also reduced (63.9%; normal range: 70%-150%). The bleeding time was slightly prolonged (8 min and 20 s; normal range: 3-7 min.) Because of coagulopathy and thrombocytopenia percutaneous liver biopsy was not performed. Biopsy of the skin showed neither vasculitis findings nor lymphoproliferative disease. An abdominal CT scan revealed a massive enlargement of the spleen (20 cm of sagittal diameter and 14 cm of antero-posterior diameter) and mild hepatomegaly without either focal lesions or abdominal lymphoadenomegaly. Thorax and neck CT scan yielded normal findings. Although in type 1 Gaucher disease the liver function tests were not so seriously altered, the presence of hepatomegaly with a progressive and massive splenomegaly together with thrombocytopenia prompted us to undergo the patient to bone morrow examination and to a measurement of acid β-glucosidase activity in circulating leukocyte cells. Although β-glucosidase activity was slightly decreased (5.5 nmol/mg/h; normal range: 8-15.1 nmol/mg/h), the bone marrow examination did not reveal the presence of the characteristics of Gaucher cells with the typical "wrinkled paper" cytoplasm. However, an intracapillary T-lymphocytes infiltration was observed. The circulating leukocytes were 14.6 × 109/L (normal range: 4.8-8.5) with absolute lymphocytosis (8.8 × 109/L, normal range: 1.6-2.4). Immunophenotype of circulating lymphocytes showed that CD3 positive lymphocytes were about 5.4 × 109/L (normal range: 1.1-1.7), CD4 positive lymphocytes were 2.5 × 109/L (normal range: 0.65-1.4), CD8 positive lymphocytes were 3.01 × 109/L (normal range: 0.32-0.90) with CD4 to CD8 ratio of 0.8% (normal range: 1%-1.5%) and CD19 positive lymphocytes were 1.23 × 109/L (normal range: 0.2-0.4). Circulating T lymphocytes (65% of overall; 5.65 × 109/L) bearing the ab type of TCR were 92.5% (normal range: 90%-95%) while circulating T lymphocytes bearing γδ TCR were about 7.5% (normal range: 5%-10%). Over half (60%) of circulating leukocytes were small to medium sized lymphoid cell with moderate amount of pale, agranular cytoplasm without villous projections. Some lymphocytes showed round or folding nucleus, with moderate clumped chromatin surrounding a small nucleolus. In spite of the opportunity to perform a transjugular liver biopsy, the presence of massive splenomegaly with pain in the left upper abdominal quadrant and early satiety, urged us to do splenectomy which was performed in April 1999. The histologic examination of the spleen revealed a hepatosplenic lymphoma. After the splenectomy, skin lesions disappeared, liver enzyme values progressively decreased and coagulation indices showed normalization in one week. As expected, platelets count increased until 738 × 109/L one week after the splenectomy and decreased to 417 × 109/L one month later. In the post surgical period, patient was treated with 4 cycles of chemotherapy (Fi2/89; epirubicine, vincristine, bleomycine and cyclophosphamide). This resulted in a complete clinical remission with negative bone marrow biopsy. In view of autologous bone marrow transplantation (ABMT) he was treated with 2 cycles of chemotherapy (BAVEC-MiMA; BiCNU, adriblastine, vepeside, vincristine, mitoxantrone, methotrexate and cytosine arabinoside). In January 2000, he was submitted to three consecutive leukapheresis but, unfortunately, the number of peripheral circulating levels of CD34 positive cells was not sufficient for the ABMT and the patient refused new leukapheresis. Two other cycles of BAVEC-MiMA chemotherapy were performed as intensive consolidation therapy after the remission. One month later (May 2000), the patient was admitted to a hematological unit because of fatigue, malaise and erythematous papules of the face and trunk similar to those that characterized the beginning of the disease. Significant laboratory values included AST 225 IU/L, ALT 255 IU/L, LDH 1773 U/L, platelets 46 × 109/L and leukocytes 27.9 × 109/L, the latter characterized by medium and large size lymphocytes with cleaved and folding nucleus. The immunophenotype of circulating lymphocytes was NK (CD16+, CD56+, CD2+, CD7+; CD8 were expressed on about 63% of NK population while CD3 was negative). He was treated with four weekly infusions of chemotherapy (MACOP-B; vincristine, adriamicine, bleomycine, cyclophosphamide, methotrexate and prednisone) with normalization of liver enzyme values, peripheral blood counts and skin lesions. The second relapse occured 10 wk later. Patient was readmitted in the same hematological unit for salvage ABMT. One-month later, he developed an acute leukemia. The patient died in December 2000, twenty months after the diagnosis. Post-mortem examination was not performed.
Informed consent for all procedures was obtained from the patient. Bone marrow specimen was fixed in 10% buffered formalin and processed according to standard technique. Paraffin sections were stained with hematoxylin-eosin, Giemsa, PAS and Gomori method. Biopsy of the skin and specimens of the spleen were fixed in 10% buffered formalin, and in B5; paraffin sections were stained with hematoxilin-eosin, Giemsa and PAS. An immunohistochemical investigation was performed on bone marrow and spleen sections according to streptavidin-biotin method. Monoclonal antibodies were used for detection of CD45, CD20, CD79a, CD10, CD45RO, CD30, CD15, CD56, CD8, CD4, TIA-1, AE1/AE3, EMA, HMB45, MIB1 antigens, and polyclonal antibodies for CD3 and S-100 protein detection. The positive reaction was revealed using diaminobenzidin as chromogen.
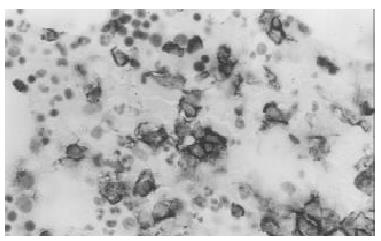
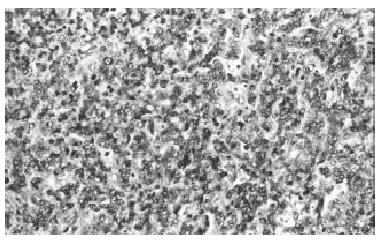
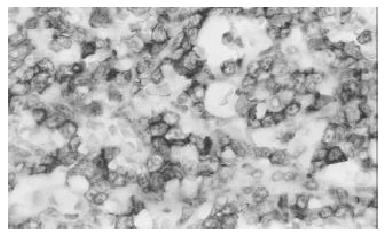
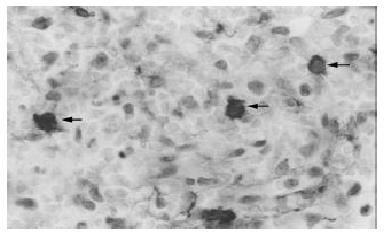
Biopsy of the skin showed a moderate aspecific lymphoid infiltration of perivascular space associated with erythrocytes. Bone marrow biopsy showed intense eritroid and megakaryocytic hyperplasia and a mild hypoplasia of myeloid compartment. Moreover an important intracapillary infiltration of medium-sized T-lymphocytes (CD45+, CD3+, scattered CD56+, CD 20-) was observed (Figure 1). The spleen weight was 3000 g. Microscopic examination showed the presence of a diffuse intrasinuses infiltrate in the red pulp of medium-sized T-lymphoid cells with round to irregular nuclei and moderately abundant pale cytoplasm (Figure 2). All neoplastic cells expressed CD45, CD45RO and CD3 membrane antigens (Figure 3), whereas they were CD4-, CD8- and TIA-1-. CD56 antigen was positive on about 50% of the T-lymphoid cells (Figure 4). The diagnosis of hepatosplenic T cell lymphoma was done. Because of the unavailability of frozen sections, the analysis of γδ TCR expression was not done.
Lymphomatous involvement of the liver has been described in three clinical situations: disseminated disease, primary liver lymphoma and hepatosplenic T-cell lymphoma. Secondary hepatic involvement by lymphoma is relatively common and indicates advanced disease. Non-Hodgkin's primary lymphoma of the liver is an uncommon entity, often clinically indistinguishable from more commonly occurring primary carcinoma and metastatic neoplasms; the correct diagnosis is based on liver biopsy or even on post-mortem examination because of lacking of suggestive clinical or imaging features. The hepatosplenic T-cell lymphoma has been recognized by Farcet et al[1] in 1990 as a distinct entity characterized by a γδ T lymphocytes sinusal infiltration of the red pulp of the spleen and by sinusoidal infiltration of the liver without lymph node involvement[2]. Because of its striking uniformity with respect to clinical, morphological and immunophenotypical features, this lymphoma was listed as a provisional entity in the Revised European-American Lymphoma (REAL) classification[3]. Later on, it has been incorporated as a distinct entity in the WHO classification of lymphoid neoplasms[4] and termed hepatosplenic γδ T-cell lymphoma. Since then, to our knowledge, 45 cases have been described[5]. According to Weidmann revision, clinical symptoms at presentation are hepato-splenomegaly, constitutional symptoms and less frequently jaundice due to hepatic involvement. The predominant laboratory findings are reduced peripheral blood cells ranging from hemolytic anemia (Coombs negative) to thrombocytopenia or pancytopenia, high levels of LDH and mild increase of liver enzymes. Moreover, recently, 14 cases of hepatosplenic lymphoma expressing αβ TCRs have been described[6].
These αβ lymphomas fulfill all the clinical, morphological and immunophenotypical criteria required for the diagnosis of hepatosplenic lymphoma, with the exception of the female preponderance (11/14 patients) and age of distribution (more wide than the γδ counterpart). Early diagnosis of this lymphoma, even if mandatory, is complicated in several cases because of the absence of lymphoadenomegaly, not specific bone marrow involvement and normal peripheral blood cell counts. The diagnosis of hepatosplenic T-cell lymphoma in the case we reported had considerable difficulties because its clinical and laboratory features were similar to acute hepatitis. As a matter of fact, the liver chemistry evaluation was typical of hepatic inflammatory disease with hepatocellular necrosis (about tenfold increase of AST and ALD and threefold increase of LDH) and impaired synthesis of liver coagulation factors whereas, the mild increase of ALP level together with normal levels of serum β-2 microglobulin and γ-GT were unusual for a lymphoproliferative disease of the liver. Probably these unusual laboratory findings were related to the quite exclusive sinusoidal infiltration by lymphoid cells without or with minimal portal involvement. However, this clinical presentation prompted us to exclude all causes of acute liver diseases such as viral hepatitis drugs, toxins, chemical ingestion and/or exposition, vascular abnormalities, autoimmune hepatitis and metabolic diseases. As the present case illustrated, an early diagnosis of hepatosplenic lymphoma was unlikely to be possible on bone marrow specimen alone. According to Galaurd[7] even when the hepatosplenic involvement is clear, the presence of few T-cells within bone marrow sinus is neither specific nor conclusive without a molecular analysis on frozen tissue. Liver biopsy, which is another way to confirm the diagnosis of hepatosplenic T-cell lymphoma, was not performed in this case because of the presence of coagulopathy and thrombocytopenia. Nevertheless, the presence of an intracapillary infiltrate by T lymphocytes on the bone marrow specimen as well as the progressive and symptomatic enlargement of the spleen prompted us to suspect the presence of a lymphoprolipherative disease and consequently to perform splenectomy. As reported in literature, splenectomy was the cornerstone for the diagnosis of hepatosplenic T-cell lymphoma. Immunophenotype studies on the spleen specimens showed the typical pattern of hepatosplenic T-cell lymphoma characterized by expression of CD45 and CD3 antigens and by double negativity for CD4 and CD8 antigens. The NK cell associated antigen CD56 expression has been reported in approximately 70%-100% of hepatosplenic T-cell lymphoma[8,9]. The presence in the bone marrow of T-lymphocytes bearing the CD56 antigen could explain the late involvement of peripheral blood by lymphoid cell with NK immunophenotype. Furthermore, in contrast with other previous investigations, in all specimens of the spleen examined, neoplastic cells were negative for TIA-1 (restricted intracellular antigen, also called granular membrane protein of 17 Kd, GMP 17), a very sensitive marker of cytosol granules independent of their activation status. Concerning the recurrence of cutaneous skin lesions, a skin biopsy, performed early in the course of disease, showed only a moderate and aspecific lymphoid infiltration of perivascular spaces, without the morphological and immunophenotypical findings that characterized the peripheral lymphoma involving the skin. Although thrombocytopenia was not severe, a complete remission of skin lesions was achieved after its correction. It seems likely to ascribe these purpuric lesions to the reduced number of circulating platelets, but a cytotoxic activity of cutaneous T lymphocytes could have a role in the pathogenesis of these skin lesions. As the present case illustrated that the prognosis for this type of lymphoma is poor. Complete remission was reported only in few patients after chemotherapy (second and third generation regime for high-grade lymphomas), followed by autologous and allogenic bone marrow or peripheral stem cell transplantation. The median survival time was 8 mo (range 0-42 month)[5].
In conclusion, the current report described the unusual clinical presentations of hepatosplenic lymphoma. This patient, in fact, shared several distinctive features including: 1) the initial clinical presentations mimicking an acute hepatitis, 2) the presence of cutaneous lesions, contemporary with the hepatosplenic involvement and 3) a negativity of TIA-1 expression of lymphoid T cells. Even in the absence of a mass lesion or lymphoadenopathy, hepatosplenic T-cell lymphoma should be included in the differential diagnosis of an acute hepatic dysfunction in young patient who shows no evidence of viral, toxic, autoimmune or metabolic liver disease.
Edited by Xu XQ and Zhu LH
| 1. | Farcet JP, Gaulard P, Marolleau JP, Le Couedic JP, Henni T, Gourdin MF, Divine M, Haioun C, Zafrani S, Goossens M. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75:2213-2219. [PubMed] |
| 2. | Gaulard P, Zafrani ES, Mavier P, Rocha FD, Farcet JP, Divine M, Haioun C, Pinaudeau Y. Peripheral T-cell lymphoma presenting as predominant liver disease: a report of three cases. Hepatology. 1986;6:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392. [PubMed] |
| 4. | Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J. Lymphoma classification--from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11 Suppl 1:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Macon WR, Levy NB, Kurtin PJ, Salhany KE, Elkhalifa MY, Casey TT, Craig FE, Vnencak-Jones CL, Gulley ML, Park JP. Hepatosplenic alphabeta T-cell lymphomas: a report of 14 cases and comparison with hepatosplenic gammadelta T-cell lymphomas. Am J Surg Pathol. 2001;25:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Gaulard P, Kanavaros P, Farcet JP, Rocha FD, Haioun C, Divine M, Reyes F, Zafrani ES. Bone marrow histologic and immunohistochemical findings in peripheral. T-cell lymphoma: A study of 38 cases. Hum Pathol. 1991;22:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Cooke CB, Krenacs L, Stetler-Stevenson M, Greiner TC, Raffeld M, Kingma DW, Abruzzo L, Frantz C, Kaviani M, Jaffe ES. Hepatosplenic T-cell lymphoma: a distinct clinicopathologic entity of cytotoxic gamma delta T-cell origin. Blood. 1996;88:4265-4274. [PubMed] |
| 9. | Salhany KE, Feldman M, Kahn MJ, Peritt D, Schretzenmair RD, Wilson DM, DiPaola RS, Glick AD, Kant JA, Nowell PC. Hepatosplenic gammadelta T-cell lymphoma: ultrastructural, immunophenotypic, and functional evidence for cytotoxic T lymphocyte differentiation. Hum Pathol. 1997;28:674-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |