Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1287
Revised: February 4, 2003
Accepted: February 11, 2003
Published online: June 15, 2003
AIM: To study the expression of IGF-1 and IGF-1R and its intervention by interleukin-10 in the course of experimental hepatic fibrosis.
METHODS: Hepatic fibrosis was induced in rats by carbon tetrachloride intoxication and liver specimens were taken from the rats administered CCl4 with or without IL-10 treatment and the animals of the control group. Immunoreactivities for insulin-like growth factor-1 (IGF-1) and IGF-1 receptor(IGF-1R) were demonstrated by immunohistochemistry, and their intensities were evaluated in different animal groups.
RESULTS: The positive levels for IGF-1 and IGF-1R were increased with the development of hepatic fibrosis, with the positive signals localized in cytoplasm and/or at the plasmic membrane of hepatocytes. The positive signals of IGF-1 and IGF-1R were observed more frequently (P < 0.01) in the CCl4-treated group (92.0% and 90.0%) compared to those in the control group. The positive signals decreased significantly (P < 0.05) in IL-10-treated group. The responses in IGF-1 and IGF-1R expression correlated with the time of IL-10 treatment.
CONCLUSION: The expression of IGF-1 and IGF-1R immunoreactivities in liver tissue seems to be up-regulated during development of hepatic fibrosis induced by CCl4, and exogenic IL-10 inhibits the responses.
- Citation: Wang XZ, Chen ZX, Zhang LJ, Chen YX, Li D, Chen FL, Huang YH. Expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor and its intervention by interleukin-10 in experimental hepatic fibrosis. World J Gastroenterol 2003; 9(6): 1287-1291
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1287.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1287
Hepatic fibrosis is a common pathological change resulted from various chronic hepatic injuries, which is characterized by an increase of extracelluar matrix (ECM) deposition in the Disse's space and the imbalance between synthesis and degeneration of ECM. It is a change before cirrhosis[1-6]. Many studies suggested that cytokines play important roles during hepatic fibrosis with different mechanisms[1,7-16]. There is a contrary effect of insulin-like growth factor-1 (IGF-1) on rat hepatic stellate cells (HSC) in vivo and in vitro. IGF-1 and its receptor (IGF-1R) may play a significant role in hepatic fibrosis. The rat hepatic fibrosis model was established and the immunoreactivities for IGF-1 and IGF-1R in rat liver tissues were assessed to show the possible involvement of IGF-1 and IGF-1R in the process of hepatic fibrosis and the effect of interleukin-10 on this change.
One hundred clean male Sprague-Dawley rats weighing 140-180 g (Provided by Shanghai Experimental Animal Center) were divided randomly into 3 groups. The control group (group C) included 24 rats; the model group (group M) included 40 rats and the IL-10 treated group (group T) included 36 rats. All the rats were bred under routine conditions.
Preparation of rats The rats of group C were injected intraperitoneally with saline 2 mL·kg-1 twice a week. The rats of group M and group T were injected intraperitoneally with 50% CCl4 (dissolved in castor oil) 2 mL·kg-1 twice a week. From the third week, the rats of group T were injected intraperitoneally with IL-10 4 μg·kg-1 (dissolved in saline) 20 min before they were injected with CCl4. All injections were given on Monday and Thursday. To the fifth week, 3 rats in group M and 2 rats in group T died; to the seventh week, 8 rats in group M and 4 rats in group T died; to the ninth week, 10 rats in group M, 6 rats in group T and 3 rats in group C died. In the 5, 7, 9 wk, 10 rats of groups M and T and 7 rats of control group were sacrificed and their livers were taken. The specimens were fixed in 10% formalin and embedded with paraffin. Sections were stained by hematoxylin and eosin and evaluated by pathologists.
Immunohistochemistry and data evaluation The rat liver tissues were sectioned at a thickness of 4 μm. The sections were deparaffinized with xylene, dehydrated with graded ethanol, incubated in PBS containing 3% H2O2 to block endogenous perxoidase activity and then incubated in PBS containing 0.1M citrate to saturate nonspecific binding sites. After incubation with rabbit anti-rat IGF-1 or IGF-1R monoclonal antibody (American Neomarkers Company), the recations were with the instance S-P immunohistochemistry reagents (American Zymed Company). And then, the sections were incubated in a buffer containing 3, 3-diaminobenzidine tetrahydrochloride (DAB) and H2O2 to produce a brown reaction product, then were dehydrated and coverslipped. The reactions were graded according to their intensities and percentage of the positive cell as follows: negative = 0, stained yellowish = 1, stained with deep yellows or brown = 2; the percentage of stained cell: < 5% = 0, 6% to 25% = 1, 26% to 50% = 2, > 50% = 3. Then the eventual result was by these two scores according to the following predefined definitions: 0 to 1 = negative (-), 2 to 3 = positive (+), 4 and above 4 = strongly positive (++). Ridit analysis described the difference between groups.
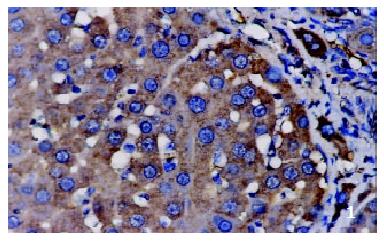
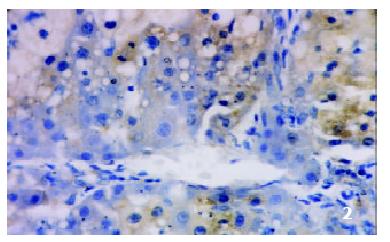
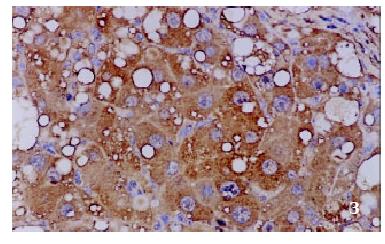
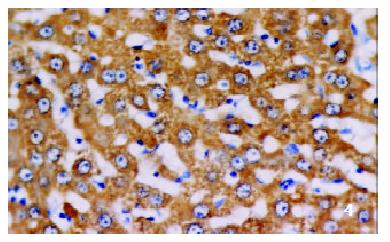
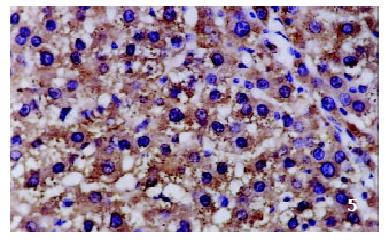
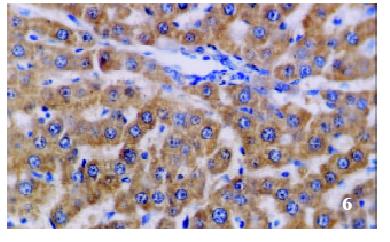
The positive rates of IGF-1 in the control group, the CCl4-treated group and the CCl4-and IL-10-treated group were 38.1%, 92.0% and 71.4%, respectively. Those of IGF-1R were 33.3%, 80.0% and 64.3%, respectively. The granular positive products were localized in the cytoplasm and/or at the membrane, but not in the nuclei. In group C, IGF-1 and IGF-1R signals were weak, mainly located in the perivenular area (Figure 1, Figure 2). In group M, the expression increased obviously with the development of hepatic fibrosis, and the positive cells distributed throughout the hepatic lobule (Figure 3, Figure 4). In group T, the changes were less pronounced than in group M (Figure 5, Figure 6).
The comparasion of IGF-1 and IGF-1R system expression levels in groups C, M and T is listed in Table 1. Ridit analysis showed a significant difference among the three groups (P < 0.01). Expression levels of the IGF-1 and IGF-1R in group M were found to be higher than that in group C (P < 0.01). In group T, after the treatment with IL-10, the immunoreactivities for IGF-1 and IGF-1R decreased (P < 0.01 and P < 0.05, respectively). The data for IGF-1 and IGF-1R reactivities in different stages of hepatic fibrosis are listed in Table 2. With the development of hepatic fibrosis, intensities of IGF-1 and IGF-1R immunoreactivities increased significantly (P < 0.05). The data for IGF-1 and IGF-1R immunoreactivities in different stages of hepatic fibrosis in group T are listed in Table 3. A significant decrease was observed in IGF-1 and IGF-1R expression with the IL-10 treatment (P < 0.05).
Hepatic fibrosis is the early stage of hepatic cirrhosis, characterized by accumulation of excessive extracellular matrix, necrosis, nodular regeneration of hepatocytes and formation of fibrous septum[1-6]. Cytokines play important roles in the formation and regression of hepatic fibrosis[1,7-16].
In the present study, up-regulated expression of IGF-1 and IGF-1R was observed in liver tissues injured by CCl4-intoxication, and was positively correlated with the development of hepatic fibrosis. However, the response was less pronounced in IL-10- treated group.
Insulin-like growth factors (IGFs) include two related homologous polypeptides: IGF-1 and IGF-2, which have similar structure and activity in vitro, but different biological effect in vivo. Activation of mitosis and induction or acceleration of differentiation are their major functions, which are mediated through IGF-1R by means of autocrine, paracrine and endocrine mechanisms. IGF-1R is a transmembrane tyrosine kinase receptor. After binding with its ligand, intracellular transcription and synthesis of proteins are activated and regulated through a series of signal transduction. This gives rise to insulin-like metabolic effects and promotes proliferation and differentiation of cells. It is also involved in the maintenance of transformed cell phenotypes. Its expression is essential for the transforming function of cell cycle-related protocogenes and viral oncogenes[17,18]. In addition, IGF-1 and IGF-1R have an anti-apoptosis effect on different cells[13]. Liver is the main organ of IGF-1, but the function of IGF-1 and IGF-1R in hepatic fibrosis still remains controversial. Many authors have observed a decreased serum concentration of IGF-1 and insulin-like growth factor-binding protein 3 (IGFBP3) in patients with hepatic cirrhosis, this change is correlative with cirrhosis progression. With the treatment of recombinant somatotropin, the serum concentration increased along with the improvement of protein synthesis and liver metabolism. For these patients, the IGF-1 concentration below 10 nmol/L was considered an unfavored response to the treatment and poor prognosis[19-31]. Castilla et al[32] and Myguerza et al[33] reported that the histological parameters in hepatic fibrosis animals were improved after being treated with exogenous IGF-1. So IGF-1 might act as an antifibrogenic factor. On the contrary, other researches speculated IGF-1 had a great influence on hepatic stellate cell (HSC), which was the main producer of ECM, such as activating proliferation, inhibiting apoptosis, accelerating the secretion of collagen type I, etc[13,34]. The function of IGF-1 also seems to be regulated by IGF-BP, IGF-1R and other cytokines[22-30].
The present study observed and evaluated IGF-1 expression with the fibrosis progression, which might be a compensatory reaction to the continuous loss of hepatocytes in the CCl4-treated animals. We consider that IGF-1 may stimulate the replication of hepatocytes and interfere with fibrosis. The discordance was observed between the IGF-1 level in liver tissue and that in serum, with the former higher and the latter lower, this is likely due to the decrease of IGF-1 released from hepatocytes to blood circulation. In other words, the hepatocytic secretion of IGF-1 maybe regulated by means of autocrine under such a situation. The positive correlation between the expression of IGF-1 and IGF-1R and the fibrosis progression may be helpful for fibrosis staging.
IL-10 is an antifibrogenic cytokine produced by Th2 cells, macrophages, stellate cells and hepatocytes[35-47]. It has been reported the deficiency of IL-10 prompted fibrosis probably by its failure in inhibiting the overproduction of TGF-β1 and TNF-α. The latter two cytokines are secreted by macrophages and can enhance synthesis of collagen type I[48]. The knock-out experiments (IL-10-/-mice) indicated that endogenous IL-10 actually relieved CCl4-induced fibrosis[49-51]. In our previous study, exogenous IL-10 was found to be able to inhibit the progress of fibrosis and might be used for treatment. Similar results were also reported by Nelson, but its mechanism remains obscure[52,53]. The present study showed that IGF-1 and IGF-1R expression decreased with the improvement of fibrosis after treatment with IL-10. It seems that antifibrogenic effect of IL-10 is associated with down-regulation of IGF-1/IGF-1R. More works are demanded to clarify whether this action is regulated by IGF-1 and IGF-1R or the decrease of IGF-1R expression is only a phenomenon of hepatic cirrhosis remission.
Edited by Su Q
| 1. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Liu HL, Li XH, Wang DY, Yang SP. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 expression in fibrotic rat liver. World J Gastroenterol. 2000;6:881-884. [PubMed] |
| 3. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I, III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. [PubMed] |
| 4. | Wang JY, Guo JS, Yang CQ. Expression of exogenous rat collagenase in vitro and in a rat model of liver fibrosis. World J Gastroenterol. 2002;8:901-907. [PubMed] |
| 5. | Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363-369. [PubMed] |
| 6. | Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol. 2001;167:7017-7026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Zhang GL, Wang YH, Teng HL, Lin ZB. Effects of aminoguanidine on nitric oxide production induced by inflammatory cytokines and endotoxin in cultured rat hepatocytes. World J Gastroenterol. 2001;7:331-334. [PubMed] |
| 8. | Daniluk J, Szuster-Ciesielska A, Drabko J, Kandefer-Szerszeń M. Serum cytokine levels in alcohol-related liver cirrhosis. Alcohol. 2001;23:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | von Baehr V, Döcke WD, Plauth M, Liebenthal C, Küpferling S, Lochs H, Baumgarten R, Volk HD. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000;47:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Si XH, Yang LJ. Extraction and purification of TGFbeta and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol. 2001;7:527-531. [PubMed] |
| 11. | Weng HL, Cai WM, Liu RH. Animal experiment and clinical study of effect of gamma-interferon on hepatic fibrosis. World J Gastroenterol. 2001;7:42-48. [PubMed] |
| 12. | Li D, Zhang LJ, Chen ZX, Huang YH, Wang XZ. Effects of TNFβ, IL-6 and IL-10 on the development of experimental rat liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2001;9:1242-1245. |
| 13. | Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Gong JP, Dai LL, Liu CA, Wu CX, Shi YJ, Li SW, Li XH. Expression of CD14 protein and its gene in liver sinusoidal endothelial cells during endotoxemia. World J Gastroenterol. 2002;8:551-554. [PubMed] |
| 15. | Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha, IFN-beta, IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999;5:38-40. [PubMed] |
| 16. | Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5:296-300. [PubMed] |
| 17. | Kalebic T, Tsokos M, Helman LJ. In vivo treatment with antibody against IGF-1 receptor suppresses growth of human rhabdomyosarcoma and down-regulates p34cdc2. Cancer Res. 1994;54:5531-5534. [PubMed] |
| 18. | Zhou P, Zhou ZC, Chen WS, Liu WW, Fang DC, Yang JM. Effect of IGF-1R antisense gene on the morphology of hepatoma cell line SMMC-7721. Shijie Huaren Xiaohua Zazhi. 2002;10:279-282. |
| 19. | Gayan-Ramirez G, van de Casteele M, Rollier H, Fevery J, Vanderhoydonc F, Verhoeven G, Decramer M. Biliary cirrhosis induces type IIx/b fiber atrophy in rat diaphragm and skeletal muscle, and decreases IGF-I mRNA in the liver but not in muscle. J Hepatol. 1998;29:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Donaghy A, Ross R, Wicks C, Hughes SC, Holly J, Gimson A, Williams R. Growth hormone therapy in patients with cirrhosis: a pilot study of efficacy and safety. Gastroenterology. 1997;113:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Møller S, Juul A, Becker U, Henriksen JH. The acid-labile subunit of the ternary insulin-like growth factor complex in cirrhosis: relation to liver dysfunction. J Hepatol. 2000;32:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Ormarsdóttir S, Ljunggren O H, Olofsson H, Blum WF, Lööf L. Circulating levels of insulin-like growth factors and their binding proteins in patients with chronic liver disease: lack of correlation with bone mineral density. Liver. 2001;21:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Assy N, Hochberg Z, Amit T, Shen-Orr Z, Enat R, Baruch Y. Growth hormone-stimulated insulin-like growth factor (IGF) I and IGF-binding protein-3 in liver cirrhosis. J Hepatol. 1997;27:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Assy N, Hochberg Z, Enat R, Baruch Y. Prognostic value of generation of growth hormone-stimulated insulin-like growth factor-I (IGF-I) and its binding protein-3 in patients with compensated and decompensated liver cirrhosis. Dig Dis Sci. 1998;43:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Shmueli E, Miell JP, Stewart M, Alberti KG, Record CO. High insulin-like growth factor binding protein 1 levels in cirrhosis: link with insulin resistance. Hepatology. 1996;24:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Møller S, Grønbaek M, Main K, Becker U, Skakkebaek NE. Urinary growth hormone (U-GH) excretion and serum insulin-like growth factor 1 (IGF-1) in patients with alcoholic cirrhosis. J Hepatol. 1993;17:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Donaghy A, Ross R, Gimson A, Hughes SC, Holly J, Williams R. Growth hormone, insulinlike growth factor-1, and insulinlike growth factor binding proteins 1 and 3 in chronic liver disease. Hepatology. 1995;21:680-688. [PubMed] |
| 28. | Møller S, Juul A, Becker U, Flyvbjerg A, Skakkebaek NE, Henriksen JH. Concentrations, release, and disposal of insulin-like growth factor (IGF)-binding proteins (IGFBP), IGF-I, and growth hormone in different vascular beds in patients with cirrhosis. J Clin Endocrinol Metab. 1995;80:1148-1157. [PubMed] [DOI] [Full Text] |
| 29. | Kratzsch J, Blum WF, Schenker E, Keller E. Regulation of growth hormone (GH), insulin-like growth factor (IGF)I, IGF binding proteins -1, -2, -3 and GH binding protein during progression of liver cirrhosis. Exp Clin Endocrinol Diabetes. 1995;103:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ottesen LH, Bendtsen F, Flyvbjerg A. The insulin-like growth factor binding protein 3 ternary complex is reduced in cirrhosis. Liver. 2001;21:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Santolaria F, González-González G, González-Reimers E, Martínez-Riera A, Milena A, Rodgíguez-Moreno F, González-García C. Effects of alcohol and liver cirrhosis on the GH-IGF-I axis. Alcohol Alcohol. 1995;30:703-708. [PubMed] |
| 32. | Muguerza B, Castilla-Cortázar I, García M, Quiroga J, Santidrián S, Prieto J. Antifibrogenic effect in vivo of low doses of insulin-like growth factor-I in cirrhotic rats. Biochim Biophys Acta. 2001;1536:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Castilla-Cortazar I, Garcia M, Muguerza B, Quiroga J, Perez R, Santidrian S, Prieto J. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology. 1997;113:1682-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Di Marco R, Xiang M, Zaccone P, Leonardi C, Franco S, Meroni P, Nicoletti F. Concanavalin A-induced hepatitis in mice is prevented by interleukin (IL)-10 and exacerbated by endogenous IL-10 deficiency. Autoimmunity. 1999;31:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Louis H, Le Moine O, Peny MO, Gulbis B, Nisol F, Goldman M, Devière J. Hepatoprotective role of interleukin 10 in galactosamine/lipopolysaccharide mouse liver injury. Gastroenterology. 1997;112:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Louis H, Le Moine O, Peny MO, Quertinmont E, Fokan D, Goldman M, Devière J. Production and role of interleukin-10 in concanavalin A-induced hepatitis in mice. Hepatology. 1997;25:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Hill DB, D'Souza NB, Lee EY, Burikhanov R, Deaciuc IV, de Villiers WJ. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2002;26:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Santucci L, Fiorucci S, Chiorean M, Brunori PM, Di Matteo FM, Sidoni A, Migliorati G, Morelli A. Interleukin 10 reduces lethality and hepatic injury induced by lipopolysaccharide in galactosamine-sensitized mice. Gastroenterology. 1996;111:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Howard M, O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 439] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 41. | de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 286] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Wakkach A, Cottrez F, Groux H. Can interleukin-10 be used as a true immunoregulatory cytokine. Eur Cytokine Netw. 2000;11:153-160. [PubMed] |
| 43. | Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30:S58-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Weng S, Leng X, Wei Y. [Interleukin-10 inhibits the activation of cultured rat hepatic stellate cells induced by Kupffer cells]. Zhonghua Yixue Zazhi. 2002;82:104-107. [PubMed] |
| 45. | Louis H, Le Moine A, Quertinmont E, Peny MO, Geerts A, Goldman M, Le Moine O, Devière J. Repeated concanavalin A challenge in mice induces an interleukin 10-producing phenotype and liver fibrosis. Hepatology. 2000;31:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Nagano T, Yamamoto K, Matsumoto S, Okamoto R, Tagashira M, Ibuki N, Matsumura S, Yabushita K, Okano N, Tsuji T. Cytokine profile in the liver of primary biliary cirrhosis. J Clin Immunol. 1999;19:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Wang XZ, Zhang LJ, Li D, Huang YH, Chen ZX, Li B. Effects of transmitters and interleukin-10 on rat hepatic fibrosis induced by CCl4. World J Gastroenterol. 2003;9:539-543. [PubMed] |
| 48. | Nagaki M, Tanaka M, Sugiyama A, Ohnishi H, Moriwaki H. Interleukin-10 inhibits hepatic injury and tumor necrosis factor-alpha and interferon-gamma mRNA expression induced by staphylococcal enterotoxin B or lipopolysaccharide in galactosamine-sensitized mice. J Hepatol. 1999;31:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Demols A, Van Laethem JL, Quertinmont E, Degraef C, Delhaye M, Geerts A, Deviere J. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol Gastrointest. Liver Physiol. 2002;282:G1105-G1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Zhang LJ, Wang XZ, Huang YH. Effect of interneukin-10 on experimental liver fibrosis. Zhonghua Xiaohuu Zazhi. 2002;22:179-180. |
| 53. | Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |