Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1270
Revised: February 4, 2003
Accepted: February 22, 2003
Published online: June 15, 2003
AIM: To explore and discuss the clinicopathologic characteristics of mucosa-associated lymphoid tissue (MALT) lymphoma in gastroscopic biopsy specimen.
METHODS: A retrospective study of 26 cases of lymphoma diagnosed by gastroscopic biopsy during 1999 to 2001 from gastroscopy files of Xijing Hospital was made. The diagnostic criteria were adopted according to the new classification of non-Hodgkin's lymphoma.
RESULTS: Twenty-six cases of primary gastric lymphoma consisting of 15 men and 11 women, aged between 23 to 76 years were recruited from 6225 cases who received gastroscopy. All of them were diagnosed by both endoscopic findings and histological examinations. Histologically, 23 cases were MALToma (low grade) and 3 cases lymphoblastic lymphoma (high grade). Immunohistochemically, all cases were CD20 positive, while CK and EMA were negative.
CONCLUSION: The majority of the cases of primary low-grade gastric lymphoma have morphologic and clinical features that justify their inclusion in the category of low-grade lymphoma of mucosa associated lymphoid tissue.
- Citation: Cheng H, Wang J, Zhang CS, Yan PS, Zhang XH, Hu PZ, Ma FC. Clinicopathologic study of mucosa-associated lymphoid tissue lymphoma in gastroscopic biopsy. World J Gastroenterol 2003; 9(6): 1270-1272
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1270.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1270
Primary gastric lymphoma is the most common extra nodal lymphoma, and the vast majority of them are of B cell origin. Most of the low-grade gastric lymphomas are of the mucosa-associated lymphoid tissue (MALT) type[1-4]. The concept of MALT lymphoma was first proposed in 1983 by Isaacson and Wright[1]. Histologically it is characterized by centrocyte-like cells, often with plasmacytic differentiation, and accompanied by lymphoepithelial lesions. The histopathological criteria for the diagnosis of gastric MALToma have largely been based on analysis of partial gastrectomy specimens. From routine biopsy diagnosis by gastroscopy, it is very difficult to distinguish MALToma from reactive lymphoid hyperplasia on small pieces of tissue. In this article, twenty-six cases were retrospectively analyzed with regard to criteria of diagnosis and clinicopathologic characteristics.
During the period of 1999-2001, 6225 cases received gastroscopy in Xijing Hospital and 26 cases of gastric lymphoma were diagnosed according to the new classification of lymphoid neoplasms. The patients (11 women and 15 men) ranged in age from 23 to 76 years. According to the new classification of lymphoid neoplasms[2], all the histology of MALToma was established if the following criteria were met: (1) invasion of epithelial structures resulting in "lymphoepithelial lesions"; (2) small lymphocytes, marginal zone cells and/or monocytoid B cells; (3) infiltration of diffuse, perifollicular, interfollicular, or even follicular type due to colonization of reactive follicles. Tissue specimens were embedded in paraffin, sectioned and stained with haematoxylin-eosin and immunohistochemical method.
Immunohistochemical staining of CD20, CD45, CD45RO, Keratin and EMA were performed on paraffin-embedded sections by using the monoclonal antibody (Dako, Copenhagen, Denmark). For antigen retrieval, the deparaffinized slides were microwaved in 0.01 mol/L sodium citrate buffer (pH6.0) for 10 min. Endogenous peroxidase activity was inhibited by hydrogen peroxidase. The sections were incubated with the monoclonal antibody (1:100) overnight at room temperature, and then the detection was performed by the avidin-biotin peroxidase complex method. The sections were counterstained with hematoxylin. PBS buffer solution was substituted for the first antibodies as the negative control, whereas the lymphoma cases were used as positive control.
There were 26 cases of MALToma, of them 15 were males and 11 females, with age ranging from 23 to 76 years. Clinical manifestations were epigastric discomfort, abdominal pain, dyspepsia, fever, melena and mucous stool. Endoscopic evaluation showed the location of 5 cases in gastric body, 4 in gastric antrum, 15 in gastric body and antrum, 1 in gastric angulus and 1 in gastric fundus. By endoscopic diagnosis, 9 cases were gastric cancer and 17 cases were gastric ulcer.
Based on the appearance of biopsy specimens, all 26 cases were gastric lymphomas, of them 23 were MALToma (low grade) and 3 lymphoblastic lymphoma (high grade).
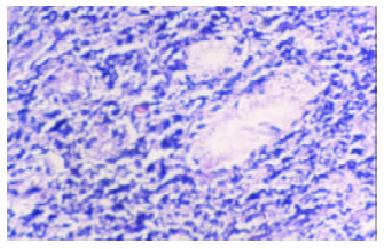
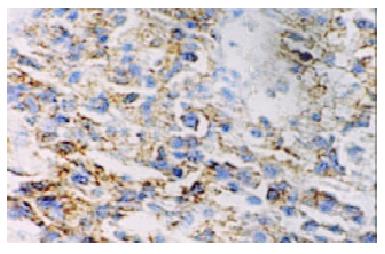
Macroscopically, MALToma could be categorized into erosion and ulcerative types; the size of tumor varied from millet to rice. The lymphomatous cells infiltrated into the mucosa, sub-mucosa and muscular layer diffusely or locally. Typical features of a low-grade mucosa-associated lymphoma with an organized architectural arrangement including lymphoid follicles, centrocyte-like cells and classical lymphoepithelial lesions were seen. Lymphoid follicles were seen and the germinal centers were partly or entirely replaced by lymphoma cells. Dendritic cells and macrophages with chromophilic bodies disappeared. This phenomenon is called follicular colonization. Lymphoepithelial lesion was shown in which there were clusters of pleomorphic, mitotically active lymphomatous cells infiltration locally and gastric glands destroyed (Figure 1). The neoplastic cells presented a serial cell lineage of small lymphocyte, centrocyte-like cell, monocyte-like B cell, lymphoplasma cell, and also centroblast like cells. In all these cells, several kinds were in a mixed distribution, but usually one kind was predominant. Immunohistochemically, all cases of MALTomas were CD20 positive (Figure 2) and CD45 positive. CK and EMA were negative in all cases.
The gastrointestinal tract is the most frequently involved extranodal site of origin for non-Hodgkin's lymphoma, accounting for 12%-15% of all non-Hodgkin's lymphomas and 30%-40% of all extranodal lymphomas[1,5]. The lymphomas in the stomach arise from MALT. Usually they are not present in the stomach but acquired following H. pylori infection of the gastric mucosa.H. pylori, a microaerophilic Gram-negative rod, has been proposed as the causative agent for low-grade primary gastric lymphoma of MALT[6-10]. Presenting features of primary gastric lymphomas are nonspecific, with dyspepsia, vague epigastric pain, nausea and vomiting[11-15]. Fever with night sweats and elevated lactate dehydrogenase levels are very uncommon. By endoscopy, diffuse gastritis, thickened gastric folds, erosions and occasionally ulcers are more common findings. Although any region of the stomach may be involved, most MALTomas arise in the antrum or the distal body, the sites commonly colonized by H. pylori. Histologically, the prominent lymphoepithelial lesion on endoscopic biopsy specimens is one of the most important features for the diagnosis of MALToma of the stomach[2,16-18]. They are seen in 100% of the biopsy specimens. It is a key point for the diagnosis of MALToma that the infiltrating lymphocytes showed homogeneity in marginal zone cells, small lymphocytes, or monocytoid B cells[2,16-18]. All of the three types of cells comprise low-grade lymphomas with a dense infiltrate of a superficial and peripheral plasma cell component. Cytologically, neoplastic lymphocytes are characterized by cellular heterogeneity, including centrocyte like cells (small, atypical cells, but with more abundant cytoplasm), monocytoid B cells, small lymphocytes, and plasma cells. Occasionally large cells (centroblast or immunoblast like) are present in most cases. If reactive follicles are present, neoplastic cells will occupy the marginal zone and/or the interfollicular region. When neoplastic lymphocytes take on a follicular pattern, it is called follicular colonization.
Therefore, the diagnosis of gastric MALToma is based on (1) prominent lymphoepithelial lesions; (2) dense lymphoid infiltrate with marginal zone cells, small lymphocytes, or monocytoid B cells. Lymphoepithelial lesions are considered as the characteristic features of MALToma. Nest formation could also be found in the inflammatory and reactive status, it might be difficult to distinguish them from the real lymphoepithelial lesions. In such cases, immunohistochemistry may be helpful. The former is several leukocytes of polyclone and the latter is the lymphocytes of mono-clone. Follicular colonization is easy to be misjudged as reactive follicles. The following morphological characteristics and immuno- phenotype may be helpful for the differentiation[14-18]: (1)Follicular colonization has no dendritic cells or macrophages with chromophilic bodies. (2) Centrocyte like cell is the immunophenotype of B cells in the marginal area, rather than at the germinal center. Both lymphoepithelial lesion and follicular colonization are characteristic features for diagnosis, but they could only be seen in some of the cases. If one case is highly suspected for MALToma of the stomach, repeated biopsy should be performed if clinically indicated and then, immunohistochemistry and molecular biology technique should be done.
Understanding primary gastric lymphomas is undergoing rapid and significant transformation from both clinicopathological and therapeutic standpoints. The most important new developments in therapy are the use of H. pylori eradication as the initial treatment for localized low-grade primary gastric lymphomas, and the use of chemoradiation over a surgical approach for localized high-grade primary gastric lymphoma[19-30]. Patients with low-grade primary gastric lymphoma should be treated with H. pylori eradication therapy as most patients respond with prompt amelioration of dyspeptic symptoms, although histological regression may take several months. Patients need to be followed-up with serial endoscopies. If no histologically proven lymphoma regression occurs, then biopsy should be done to rule out the presence of co-existing high-grade primary gastric lymphoma. The finding of high-grade primary gastric lymphoma usually indicates the need for more aggressive treatment with chemotherapy and radiotherapy. Surgery is an option in cases of significant hemorrhage or perforation. With appropriate treatment, the long-term survival is 70%-80% for limited-stage disease and 50%-60% for advanced stage disease.
Edited by Xu JY
| 1. | Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A. Helicobacter and gastric MALT lymphoma. Gut. 2002;50 Suppl 3:III19-III24. [PubMed] [Cited in This Article: ] |
| 2. | Isaacson PG, Muller-Hermelink HK, Paris MA, Berger F, Nathwani BN, Swerdlow SH, Harris NL. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In Jaffe ES, Harris NL, Stein H, Vardiman JW editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press 2001; 157-160. [Cited in This Article: ] |
| 3. | Zhou Q, Xu TR, Fan QH, Zhen ZX. Clinicopathologic study of primary intestinal B cell malignant lymphoma. World J Gastroenterol. 1999;5:538-540. [PubMed] [Cited in This Article: ] |
| 4. | Salam I, Durai D, Murphy JK, Sundaram B. Regression of primary high-grade gastric B-cell lymphoma following Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2001;13:1375-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Delchier JC, Lamarque D, Levy M, Tkoub EM, Copie-Bergman C, Deforges L, Chaumette MT, Haioun C. Helicobacter pylori and gastric lymphoma: high seroprevalence of CagA in diffuse large B-cell lymphoma but not in low-grade lymphoma of mucosa-associated lymphoid tissue type. Am J Gastroenterol. 2001;96:2324-2328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Go MF, Smoot DT. Helicobacter pylori, gastric MALT lymphoma, and adenocarcinoma of the stomach. Semin Gastrointest. Dis. 2000;11:134-141. [PubMed] [Cited in This Article: ] |
| 7. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1504] [Cited by in F6Publishing: 1354] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 8. | Furusyo N, Kanamoto K, Nakamura S, Yao T, Suekane H, Yano Y, Ariyama I, Hayashi J, Kashiwagi S. Rapidly growing primary gastric B-cell lymphoma after eradication of Helicobacter pylori. Intern Med. 1999;38:796-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Montalban C, Santon A, Boixeda D, Bellas C. Regression of gastric high grade mucosa associated lymphoid tissue (MALT) lymphoma after Helicobacter pylori eradication. Gut. 2001;49:584-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Miki H, Kobayashi S, Harada H, Yamanoi Y, Uraoka T, Sotozono M, Ohmori M. Early stage gastric MALT lymphoma with high-grade component cured by Helicobacter pylori eradication. J Gastroenterol. 2001;36:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Gretschel S, Hünerbein M, Foss HD, Krause M, Schlag PM. Regression of high-grade gastric B-cell lymphoma after eradication of Helicobacter pylori. Endoscopy. 2001;33:805-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248-253. [PubMed] [Cited in This Article: ] |
| 13. | Morgner A, Miehlke S, Fischbach W, Schmitt W, Müller-Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer A, Stolte M. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol. 2001;19:2041-2048. [PubMed] [Cited in This Article: ] |
| 14. | Bouzourene H, Haefliger T, Delacretaz F, Saraga E. The role of Helicobacter pylori in primary gastric MALT lymphoma. Histopathology. 1999;34:118-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdörffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Tang CC, Shih LY, Chen PC, Chen TC. Simultaneous occurrence of gastric adenocarcinoma and low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Chang Gung Med J. 2002;25:115-121. [PubMed] [Cited in This Article: ] |
| 18. | Papa A, Cammarota G, Tursi A, Gasbarrini A, Gasbarrini G. Helicobacter pylori eradication and remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma: a long-term follow-up study. J Clin Gastroenterol. 2000;31:169-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] [Cited in This Article: ] |
| 20. | Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103-1107. [PubMed] [Cited in This Article: ] |
| 21. | Lu XL, Qian KD, Tang XQ, Zhu YL, Du Q. Detection of H. pylori DNA in gastric epithelial cells by in situ hybridization. World J Gastroenterol. 2002;8:305-307. [PubMed] [Cited in This Article: ] |
| 22. | Vandenplas Y. Helicobacter pylori infection. World J Gastroenterol. 2000;6:20-31. [PubMed] [Cited in This Article: ] |
| 23. | Hu PJ. Hp and gastric cancer: challenge in the research. Shijie Huaren Xiaohua Zazhi. 1999;7:1-2. [Cited in This Article: ] |
| 24. | Quan J, Fan XG. Progress in experimental research of Helicobacter pylori infection and gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:1068-1069. [Cited in This Article: ] |
| 25. | Guo CQ, Wang YP, Ma SW, Ding GY, Li LC. Study on Helicobacter pylori infection and p53, c-erbB-2 gene expression in carcinogenesis of gastric mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:313-315. [Cited in This Article: ] |
| 26. | Xiao SD, Liu WZ. Current status in treatment of Hp infection. Shijie Huaren Xiaohua Zazhi. 1999;7:3-4. [Cited in This Article: ] |
| 27. | Begum S, Sano T, Endo H, Kawamata H, Urakami Y. Mucosal change of the stomach with low-grade mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori: follow-up study of 48 cases. J Med Invest. 2000;47:36-46. [PubMed] [Cited in This Article: ] |
| 28. | Yamashita H, Watanabe H, Ajioka Y, Nishikura K, Maruta K, Fujino MA. When can complete regression of low-grade gastric lymphoma of mucosa-associated lymphoid tissue be predicted after helicobacter pylori eradication. Histopathology. 2000;37:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Urakami Y, Sano T, Begum S, Endo H, Kawamata H, Oki Y. Endoscopic characteristics of low-grade gastric mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2000;15:1113-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Alpen B, Thiede C, Wündisch T, Bayerdörffer E, Stolte M, Neubauer A. Molecular diagnostics in low-grade gastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type after Helicobacter pylori eradication therapy. Clin Lymphoma. 2001;2:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |