Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1241
Revised: February 4, 2003
Accepted: February 15, 2003
Published online: June 15, 2003
AIM: TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) has been reported to specifically induce apoptosis of cancer cells although only a small percentage of cell lines were sensitive to it. Cell lines not responding to TRAIL in vitro were said to be more prone to apoptosis when TRAIL was combined with another anticancer agent. Generally, factors affecting drug-sensitivity involve many apoptosis-related proteins, including p53. The expression of wild-type p53 gene was proposed as an important premise for tumor cells responding to chemotherapy. The present study was to investigate the cell killing action of TRAIL on colon cancer cell line SW480, its synergistic effect with doxorubicin, and the possible mechanisms.
METHODS: SW480 cells were cultured in the regular condition and incubated with different levels of agents. Morphologic changes in these cells after treatment were observed under phase-contrast microscope and cytotoxicity by TRAIL alone and in combination with doxorubicin was quantified by a 1-day microculture tetrazolium dye (MTT) assay. In addition, flow cytometry assay (FCM) and transmission electron microscopy were used to detect apoptosis among these cells. Variation of p53 protein level among different groups according to concentrations of agents was measured by Western blot assay.
RESULTS: (1) SW480 cells were not sensitive to TRAIL, with IC50 > 1 mg•l-1 and dose-independent cytotoxicity. (2) SW480 cells were sensitive to doxorubicin at a certain degree, with dose-dependent cytotoxicity and IC50 = 65.25 ± 3.48 μmol•l-1. (3) TRAIL could synergize with doxorubicin to kill SW480 cells effectively, which was represented by the boosted killing effect of doxorubicin on theses cells. IC50 of doxorubicin against SW480 cells sharply reduced when it was combined with TRAIL. (4) Subtoxic TRAIL (100 μg•l-1), combined with subtoxic doxorubicin (0.86 μmol•l-1), could kill SW480 cells sufficiently. Cytotoxicity by MTT assay arrived at 80.12% ± 2.67%, which was significantly higher than that by TRAIL or doxorubicin alone, with P = 0.006 and 0.003 respectively. This killing effect was partly due to apoptosis. It was proved by large amounts of apoptotic cells under phase-contrast microscopy, cell apoptosis rate of 76.82% ± 1.93% by FCM assay and typical apoptotic morphology observed through transmission electron microscopy. Increase of apoptosis after combined treatment had no relation with protein level of p53 (P > 0.05).
CONCLUSION: SW480 cells are not sensitive to TRAIL, but TRAIL can synergize with lower concentration of doxorubicin to induce apoptosis effectively. The status of p53 protein is not involved in the mechanism of synergistic apoptosis. It suggests the potential therapeutic applicability of the combination of TRAIL with doxorubicin against colon cancers.
- Citation: Xu LH, Deng CS, Zhu YQ, Liu SQ, Liu DZ. Synergistic antitumor effect of TRAIL and doxorubicin on colon cancer cell line SW480. World J Gastroenterol 2003; 9(6): 1241-1245
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1241.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1241
The rapid induction of apoptosis by TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) preferentially in tumor cells but not in normal cells has implied its potential use in therapy of malignancies[1,2]. Recent studies indicated that many cancer cells in vitro were resistant to apoptosis-inducing effect of TRAIL[3,4]. Enhancement of the antitumor activity of TRAIL by metabolic inhibitors has led to later studies encompassing the augmentation of antitumor effects of several kinds of chemotherapy or radiation therapy by TRAIL[5-13].
Doxorubicin has been the regular chemotherapeutic agents for its strong cell killing ability. But severe systemic toxicity limited its use in the clinical treatment of cancer. Reduction of its dose and maintenance of its high efficacy will be necessary in the future treatment of tumors. More efforts have been made to explore the combination of chemodrugs with some other agents[14].
SW480 cell line was purchased from ATCC and maintained in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco) supplemented with 2 mmol•l-1 L-glutamine (Gibco), 100 kU•l-1 penicillin (Gibco), 100 g•l-1 streptomycin (Gibco) and 100 mL•l-1 heat-inactivated fetal bovine serum (Gibco), hereafter referred to as complete medium. Recombinant human soluble TRAIL (rhsTRAIL) was purchased from Pepro Tech, USA. Doxorubicin, p53 Mab and MTT were respectively from Sigma Co, Santa Cruz Company and Amresco Co.
Cytotoxicity assay Cytotoxicity was determined by MTT assay. In brief, the cells were cultured in 96-well microtiter plates under 50 mL•l-1 CO2 in a humidified atmosphere at 37 °C. Each well was then incubated for 4 h with MTT solution (final concentration 0.45 g•l-1, Sigma Chemical, St.Louis, MO) under the same condition. Culture medium in each well was discarded and replaced with 100 μL of dissolving solution (DMSO). The absorbance of each well was determined by a spectrophotometer with a 490 nm wavelength. The percentage of cell viability was calculated by multiplying the ratio absorbance of the sample versus the control by 100. Doxorubicin IC50 was determined as a doxorubicin concentration showing 50% cell growth inhibition as compared with control cell growth.
Antitumor activity of doxorubicin and/or TRAIL The cells were placed at 4 × 104 cells/well in 96-well microtiter plates. After 24 h culture for cell adherence to the plate, doxorubicin and/or TRAIL were added to each well at a given concentration. Detection of cell viability by MTT assay was performed following further incubation of the cells with doxorubicin and/or TRAIL for another 24 h. One well treated by the complete medium with neither doxorubicin nor TRAIL served as a control. The experiments were repeated in triplicate, and the percentage of cell viability was expressed as mean ± standard deviation.
Flow cytometry This analysis was conducted with a Facscan flow cytometer (Becton Dickinson, Mountain View, CA). It was to quantify cell apoptosis by the propidium iodide method. In brief, the cells were harvested by trypsinization at 24 h after treatment and washed twice with PBS (pH7.2). The medium and PBS were placed in tubes and centrifuged. A total of 1 mL of hypotonic buffer (propidium iodide, 50 mg•l-1, in 1 mL•l-1 sodium citrate, Rnase plus 1 mL•l-1 Triton X-100; sigma) was added directly to the tubes and gently pipetted off. The tubes were placed at 4 °C in the dark for half an hour before flow cytometry analysis. The propidium iodide fluorescence of individual nuclei was measured in the red fluorescence and the data were registered in a logarithmic scale. At least 104 cells of each sample were analyzed each time. Apoptotic nuclei appeared as a broad hypodiploid DNA peak before the G1 phase of cell cycle, which was easily distinguished from the narrow hyperdiploid DNA peak at the G1 phase or later G2 and S phase.
Transmission electron microscopy Transmission electron microscopy was used to observe apoptosis directly through morphological changes at the subcellular level. In brief, the cells were cultured in the regular condition for 24 h. Then, TRAIL (100 μg•l-1), doxorubicin (0.86 μmol•l-1), their combination or the complete culture medium was added to them and maintained for another 24 h. After these, the cells were subsequently trypsinized, harvested, fixed in glutaral, immersed with Epon 821, imbedded in capsules and converged. Ultrathin sections of 60 nm were finally prepared and stained with uranyl acetate and lead citrate. Cell morphology was measured by transmission electron microscope.
Western blot analysis for p53 protein expression p53 levels following treatment with doxorubicin and/or TRAIL were investigated by Western blot analysis. The cells were incubated with the medium alone, doxorubicin alone, TRAIL alone, or the combination of doxorubicin and TRAIL at 37 °C for 24 h. The cells were lysed in a lysis buffer consisting of 1 mmol•l-1dithiothreitol, 1 mmol•l-1 phenylmethyl sulphonyl fluoride, 5 mg•l-1 leupiptin, 1 mmol•l-1 NaF, 10 mmol•l-1β-glycerophosphate, 2 mmol•l-1 Natrium orthovanadate, 50 mmol•l-1 HEPES, 250 mmol•l-1 NaCl, 1 mmol•l-1 EDTA, and 1% Nonidet P-40. After centrifuged for 7 min at 14000 g, the supernatant was collected and the total protein amount was determined by SPECTRAmax 250. Twelve mg/lane of proteins were boiled in a sample buffer for 1 min at 100 °C, electrophoresed in a multigel 10/20, and then transferred to Immobilon-P membranes (Millipore). Nonspecific antibody bindings were blocked by preincubation of the membranes for 1 h with a 5% skim milk suspension. Then the membranes were incubated for 1 h with the polyclonal rabbit anti-human p53 antibody (1:200) (Santa Cruz, Delaware, CA), followed by further incubation overnight with horseradish peroxidase conjugated anti-rabbit IgG (1:2000) (Santa Cruz). After each antibody incubation, the membranes were washed three times by Tris-buffer saline. To visualize the protein bands, ECL Western blot detection reagents (Amersham, Buckinghamshire, UK) were used and the membranes were exposed to X-ray film.
All determinations were made in triplicate, and the results were expressed as the mean standard deviation (S.D.). Statistical significance was determined by Student's t-test. A P value of 0.05 or less was considered significant.
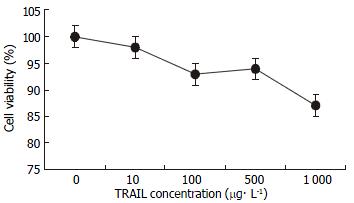
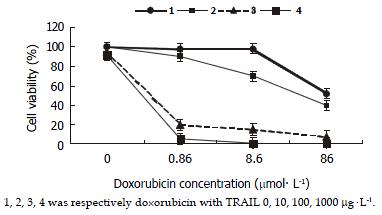
MTT assay demonstrated that SW480 cells were not sensitive to TRAIL. No dose-dependent cytotoxicity of TRAIL on these cells was found at the concentrations from 0 μg•l-1, 10 μg•l-1, 100 μg•l-1, 500 μg•l-1, to 1 mg•l-1, (as shown in Figure 1). IC50was above 1 mg•l-1 which was significantly higher than 0.4 μg•l-1 of TRAIL-sensitive leukaemic cell line Jurkat. 100 μg•l-1TRAIL could only affect 5.8% of these cells, a subtoxic concentration. By contrast, SW480 cells responded to doxorubicin sensitively, with obvious dose-dependent cytotoxicity and IC50 of 65.25 ± 3.48 μmol•l-1. 0.86 μmol•l-1 doxorubicin could not damage any cell, also a subtoxic concentration (as shown in Figure 2). All values by MTT assay were proved by flow cytometry assay and apoptosis applauded parallel with cytotoxicity.
SW480 cells were treated by doxorubicin alone, TRAIL alone, and a combination of doxorubicin and TRAIL, and cell viability after each treatment was determined by MTT assay. The cell growth inhibitory effects of the combined treatment were synergistic and more significant in proportion to the increasing concentrations of TRAIL as compared with doxorubicin alone. Furthermore, TRAIL could cause a decrease in doxorubicin IC50 (as shown in Figure 2).
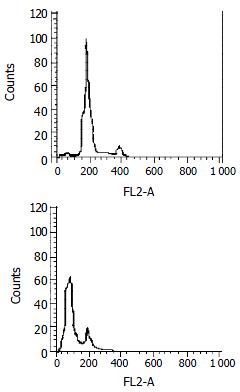
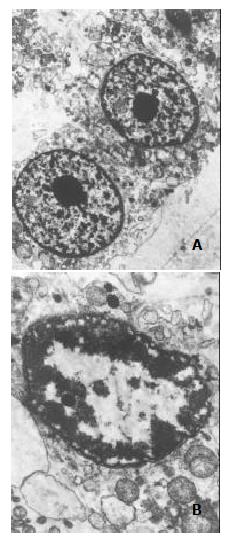
Subtoxic concentration TRAIL of 100 μg•l-1, with subtoxic concentration doxorubicin of 0.86 μmol•l-1 was capable of killing 80.12% ± 2.67% cells by MTT assay. Much more apoptotic cells were observed under phase-contrast microscope in the group treated by this combination compared with those treated by TRAIL or doxorubicin alone. Flow cytometry assay also measured apoptosis rate of 76.82% ± 1.93% and obvious pre-G1 apoptotic DNA peak among the cells (as shown in Figure 3). And electron microscopy observed a large number of apoptosis cells in them (as shown in Figure 4).
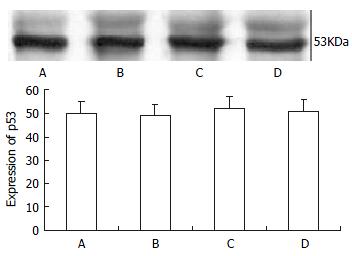
SW480 cells were treated with 100 μg•l-1 TRAIL alone, 0.86 μmol•l-1 doxorubicin alone, and their combination or complete control medium for 24 h. The protein level of mutant p53 was not different among them, with P > 0.05 (as shown in Figure 5). Variation of agent concentration turned out similar result.
TRAIL, also named Apo-2L, is a new member of the TNF cytokine family. It resembles Fas/Apo-2L in its amino acid sequence, as well as in its ability to induce apoptosis. It conducts or regulates the activities via a set of receptors on the surface of various cells[15-24]. TRAIL has attracted much interest for a profound feature since its discovery in 1995. That is the selectivity of killing cells, namely, TRAIL only induces apoptosis in transformed or tumor cells, but does not damage the normal ones[1,2]. There was a proposal for its clinical use after a series of in vitro experimentations. But a variety of cancer cells of breast, lung, prostate, colon and bladder were reported resistant to the effect of TRAIL[5-7].
Results in this study demonstrated that colon cancer cell line SW480 was relatively resistant to TRAIL, compared with TRAIL-sensitive leukaemic cell line Jurkat. The mechanisms of the underlying resistance was not clear update[7-9]. The conditions of decoy receptors, caspase-inhibition protein (FLIP), caspases and some apoptosis-related genes might attribute to the different responses[25-34]. It's necessary to find a way to reverse the resistance if it is used in clinic in the future. Previous articles indicated some chemotherapeutic drugs could sensitize the cancer cells to TRAIL-mediated apoptosis[10-15]. Doxorubicin is a member of antibiotics, specifically for cancer cells. It belongs to the classical chemotherapeutic agents, with high anti-cancer efficacy. However, severe cardiac toxicity limits its broad use in clinic. Reducing concentration and maintaining efficacy of doxorubicin should be necessary for continuing its work. This study also demonstrated the sensitivity of SW480 cells to doxorubicin and its synergistic effect with TRAIL when percentage of cell viabilities were significantly decreased by the combined treatment at any dose as compared with those treated by doxorubicin or TRAIL alone. The degrees of growth inhibition were greater accompanied by the increased concentrations of TRAIL. Furthermore, augmentation of the cytotoxic effect of doxorubicin with TRAIL was significant even at low Doxorubicin (0.86 μmol•l-1) and low TRAIL (100 μg•l-1) concentrations in SW480 cells. It presented the evidence that TRAIL could reduce the dose of doxorubicin against tumor cells, which ultimately resulted in minimizing risks for systemic side effects while increasing the efficacy of doxorubicin, suggesting the clinical applicability of this combination for colon cancers.
Concentrations of TRAIL that were completely inactive on their own, boosted the activity of doxorubicin in the SW480 cells. This correlated well with the increased ability of this drug when combined with TRAIL to induce apoptosis and it did not correlate with the status of p53. Mutant-type p53 was said to contribute to drug-resistance of most cancer cells[27,28]. The precise mechanisms enabling TRAIL to augment the cytotoxicity of chemotherapeutic agents in the p53 mutant-type cells have not been extensively investigated; however, recent evidence has suggested several possibilities: First, the augmentation of TRAIL-induced apoptosis by adriamycin or 5FU in p53 wild- and mutant-type breast cancer cell lines was mediated through a selective activation of caspases by these agents[28]. Subsequent investigations demonstrated that caspase activation, which was not observed with TRAIL or doxorubicin alone, became more evident after the combined treatment in p53 mutant-type cell lines. These findings implied that the up-regulation of intracellular apoptotic signaling events contributed to overcome resistance to TRAIL alone even in p53 mutant-type cells. Second, the increase of KILLER/DR5 expression by certain genotoxic stresses, which resulted in the enhancement of TRAIL, is regulated in a p53-dependent and -independent manner[29,30]. Finally, another TRAIL receptor, DR4, which transmitted a death signal through its binding with TRAIL, is regulated in a p53-independent manner[31-34].
The finding that TRAIL could boost the activity of doxorubicin against colorectal carcinoma cells should have important therapeutic implications. Since TRAIL itself is thought to have no apparent toxicity to normal cells and to be safe in systemic administration, the combination of doxorubicin and TRAIL will result in minimization of the toxicity and maximization of the antitumor activity.
Edited by Wu XN
| 1. | Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1724] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 2. | Lin T, Huang X, Gu J, Zhang L, Roth JA, Xiong M, Curley SA, Yu Y, Hunt KK, Fang B. Long-term tumor-free survival from treatment with the GFP-TRAIL fusion gene expressed from the hTERT promoter in breast cancer cells. Oncogene. 2002;21:8020-8028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Jang YJ, Park KS, Chung HY, Kim HI. Analysis of the phenotypes of Jurkat clones with different TRAIL-sensitivities. Cancer Lett. 2003;194:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Naumann U, Waltereit R, Schulz JB, Weller M. Adenoviral (full-length) Apo2L/TRAIL gene transfer is an ineffective treatment strategy for malignant glioma. J Neurooncol. 2003;61:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999;59:6153-6158. [PubMed] |
| 6. | Jin Z, Dicker DT, El-Deiry WS. Enhanced sensitivity of G1 arrested human cancer cells suggests a novel therapeutic strategy using a combination of simvastatin and TRAIL. Cell Cycle. 2002;1:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Hernandez A, Wang QD, Schwartz SA, Evers BM. Sensitization of human colon cancer cells to TRAIL-mediated apoptosis. J Gastrointest. Surg. 2001;5:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Hietakangas V, Poukkula M, Heiskanen KM, Karvinen JT, Sistonen L, Eriksson JE. Erythroid differentiation sensitizes K562 leukemia cells to TRAIL-induced apoptosis by downregulation of c-FLIP. Mol Cell Biol. 2003;23:1278-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734-741. [PubMed] |
| 10. | Vignati S, Codegoni A, Polato F, Broggini M. Trail activity in human ovarian cancer cells: potentiation of the action of cytotoxic drugs. Eur J Cancer. 2002;38:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Mizutani Y, Nakanishi H, Yoshida O, Fukushima M, Bonavida B, Miki T. Potentiation of the sensitivity of renal cell carcinoma cells to TRAIL-mediated apoptosis by subtoxic concentrations of 5-fluorouracil. Eur J Cancer. 2002;38:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Shimoyama S, Mochizuki Y, Kusada O, Kaminishi M. Supra-additive antitumor activity of 5FU with tumor necrosis factor-related apoptosis-inducing ligand on gastric and colon cancers in vitro. Int J Oncol. 2002;21:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Wei XC, Wang XJ, Chen K, Zhang L, Liang Y, Lin XL. Killing effect of TNF-related apoptosis inducing ligand regulated by tetracycline on gastric cancer cell line NCI-N87. World J Gastroenterol. 2001;7:559-562. [PubMed] |
| 14. | Chen XX, Lai MD, Zhang YL, Huang Q. Less cytotoxicity to combination therapy of 5-fluorouracil and cisplatin than 5-fluorouracil alone in human colon cancer cell lines. World J Gastroenterol. 2002;8:841-846. [PubMed] |
| 15. | Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1291] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 16. | Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;7:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 547] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 17. | Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386-5397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 877] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 18. | Sträter J, Hinz U, Walczak H, Mechtersheimer G, Koretz K, Herfarth C, Möller P, Lehnert T. Expression of TRAIL and TRAIL receptors in colon carcinoma: TRAIL-R1 is an independent prognostic parameter. Clin Cancer Res. 2002;8:3734-3740. [PubMed] |
| 19. | Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 486] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 20. | Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 643] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 21. | Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 490] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998;424:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 233] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363-14367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 879] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 24. | Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912-916. [PubMed] |
| 25. | Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 348] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Grotzer MA, Eggert A, Zuzak TJ, Janss AJ, Marwaha S, Wiewrodt BR, Ikegaki N, Brodeur GM, Phillips PC. Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene. 2000;19:4604-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Xu SQ, El-Deiry WS. p21(WAF1/CIP1) inhibits initiator caspase cleavage by TRAIL death receptor DR4. Biochem Biophys Res Commun. 2000;269:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Xu M, Jin YL, Fu J, Huang H, Chen SZ, Qu P, Tian HM, Liu ZY, Zhang W. The abnormal expression of retinoic acid receptor-beta, p 53 and Ki67 protein in normal, premalignant and malignant esophageal tissues. World J Gastroenterol. 2002;8:200-202. [PubMed] |
| 29. | Siervo-Sassi RR, Marrangoni AM, Feng X, Naoumova N, Winans M, Edwards RP, Lokshin A. Physiological and molecular effects of Apo2L/TRAIL and cisplatin in ovarian carcinoma cell lines. Cancer Lett. 2003;190:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Ng CP, Zisman A, Bonavida B. Synergy is achieved by complementation with Apo2L/TRAIL and actinomycin D in Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: role of XIAP in resistance. Prostate. 2002;53:286-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Lee KY, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Exp Mol Med. 2002;34:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Kelly MM, Hoel BD, Voelkel-Johnson C. Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer Biol Ther. 2002;1:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Thomas RP, Farrow BJ, Kim S, May MJ, Hellmich MR, Evers BM. Selective targeting of the nuclear factor-kappaB pathway enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated pancreatic cancer cell death. Surgery. 2002;132:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Shetty S, Gladden JB, Henson ES, Hu X, Villanueva J, Haney N, Gibson SB. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) up-regulates death receptor 5 (DR5) mediated by NFkappaB activation in epithelial derived cell lines. Apoptosis. 2002;7:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |