Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1196
Revised: March 1, 2003
Accepted: March 3, 2003
Published online: June 15, 2003
AIM: To identify the gene that may predispose to human gastric cancer and to analyze its expression in gastric cancer and non-tumorous gastric mucosa.
METHODS: Cancer, para-tumor, and non-tumor gastric tissues were studied for gene expression profile using fluorescent differential display reverse transcription polymerase chain reaction (DDRT-PCR). The differentially expressed bands of interest were analyzed by cloning, Northern blotting, and sequencing. The sequencing results were compared with the GenBank database for homology and conserved domain analysis. In situ hybridization with DIG-labeled cRNA probes was used to detect the expression of gene in paraffin embedded gastric adenocarcinoma and non-cancerous tissues.
RESULTS: A gene expressed higher in tumor and para-tumor tissues than in their non-tumor counterparts of all 7 tested gastric adenocarcinoma patients was identified by means of DDRT-PCR analysis. It was named GCRG213 (gastric cancer related gene 213). Northern blot confirmed the differential expression. GCRG213 (GenBank No. AY053451) consisted of 1094 base pairs with an open reading frame (ORF) which encoded 142 amino acids. The deduced amino acid sequence contained a putative conserved domain, apurinic/apyrimidinic endonuclease (APE). In situ hybridization analysis showed that GCRG213 was expressed higher in gastric cancer tissues than in their corresponding non-tumor ones. Precancerous leisions of gastric adenocarcinoma showed a high GCRG213 expression, too. No difference of the expression patterns was found between the early and advanced gastric cancer.
CONCLUSION: A gene named GCRG213 was identified in human gastric adenocarcinoma. It encoded an APE-like protein which was probably a new member of the APE family. GCRG213 was over-expressed not only in gastric cancer, but also in its precancerous leisions. The role of GCRG213 expression in carcinogenesis needs further study.
- Citation: Wang GS, Wang MW, Wu BY, Liu XB, You WD, Yang XY. A gene encoding an apurinic/apyrimidinic endonuclease-like protein is up-regulated in human gastric cancer. World J Gastroenterol 2003; 9(6): 1196-1201
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1196.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1196
Gastric cancer is the second most common cause of cancer-related deaths in the world. It is widely accepted that genetic alterations play an important role in the pathogenesis of gastric cancer[1,2]. The expression of oncogenes such as c-met, c-myc, ras, c-erbB-2[3-5], the inactivation of tumor-suppressor genes, such as p53, p16, Rb, DCC, APC[6-12]; and the abnormal transcription of genes related to metastasis, such as nm23, CD44, and E-cadherin[12,13], have been reported in patients with gastric adenocarcinoma. Recent data showed that cancer related genes such as COX-2[14,15], survivin[16,17], metallothionein II and RUNX3, etc.[18-22] were also expressed abnormally in gastric cancer. With the development of molecular biology techniques, such as cDNA array and differential display reverse transcription polymerase chain reaction (DDRT-PCR), some novel genes or cDNA fragments closely related to the development of human gastric cancer were identified recently[23-32]. However, the genetic factors in human gastric cancer and their mechanisms of carcinogenesis remain uncertain and deserve further study. We, therefore, used DDRT-PCR to screen the human intestinal-type gastric adenocarcinoma and its precursor lesions for the differential expression of gastric cancer related genes (GCRGs).
In our previous reports, we described a cDNA fragment which was upregulated in human gastric cancer tissues. Two subclones, GCRG213 and GCRG224, were subsequently identified. GCRG224 was overexpressed in almost all gastric mucosal epithelia but only in a small portion of gastric cancer and precancerous leisions[33]. In this study, we investigated the subclone, GCRG213 and found that it was a gene encoding an apurinic/apyrimidinic endonucleas1(APE)-like protein. GCRG213 was overexpressed in gastric cancer and in its precancerous leisions.
Fresh primary intestinal-type gastric adenocarcinoma, para-tumor tissues and non-cancerous gastric mucosal tissues were collected from 7 patients (male: 4, female: 3; mean age: 51 ± 18 years old) during surgical operation for the differential display analyses of genes. The para-tumor tissues were collected at 1.0 cm away from the tumor mass. Three other sets of fresh gastric adenocarcinoma and non-cancerous gastric tissues were used for Northern blot analysis. Specimens of paraffin embedded gastric adenocarcinoma (male: 21, female: 11; mean age 57 ± 8 years old) and their corresponding noncancerous tissues were collected for in situ hybridization analysis. Of the 32 cases of gastric adenocarcinoma, 15 cases were early gastric cancer while the other 17 cases were advanced carcinoma. The diagnosis of cancer was confirmed through histological findings.
Total RNA was extracted from tissues using TRIzol reagent (Life Technologies, Inc., Rockville, Maryland) according to the instructions of the manufacturer. The fluorescent differential display was performed as previously described[34]. The primers used in the assay were T12GG vs. ARP-8: 5'-TGGTAAAGGG-3' (Genomyx Corporation, Foster City, CA). The intensity of differentially expressed bands was quantified by Image Quant software (Molecular Dynamics, Sunnyvale, CA). The differentially expressed cDNA fragments were sub-cloned and sequenced as described previously[33]. The sequenced cDNA was analyzed via the BLAST program for matches in the GenBank database[35], and DNASIS software (Hitachi Software Engineering America Ltd., San Bruno, CA) was used for bioinformatic analysis.
Dig Northern Starter Kit (Roche Diagnostic Corporation, Indianapolis, IN) was used. The procedure of hybridization was performed according to the manufacturer's protocol. Anti-sense cRNA probe labeled with digoxigenin was generated from a digested cDNA insert by means of in vitro transcription. Digoxigenin labeled sense cRNA probe was used as a negative control. The hybridization signals were visualized with chemiluminescence which is recorded on X-ray films. The exposure time was 10 min.
All specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. A series of 5 μm thick sections were cut for analysis. In situ hybridization (ISH) was performed as previously described[36,37] using anti-sense cRNA probe labeled with digoxigenin. Briefly, the slides were dried at 40 °C overnight, dewaxed, rehydrated and pretreated with DEPC-treated PBS containing Triton X-100. The sections were then permeabilized with 20 μg/mL RNase-free proteinase K (Merck, Darmstadt, Germany) for 20 min, incubated at 37 °C for at least 20 min with prehybridization buffer. Each section was overlaied with 30 μL of hybridization buffer containing 10 ng of digoxigenin-labeled cRNA probe and incubated at 42 °C overnight. The positive signal for GCRG213 mRNA was finally detected by using NBT/BCIP as substrate. Sense cRNA probes were used as negative control. The presentation of blue staining in cytoplasm was considered positive. The positive staining of cytoplasm was semi-quantified as Grade: barely detectable light blue, Grade 1+: diffuse light blue, Grade 2+: blue staining, and Grade 3+: dark blue. More than 100 non-tumor or tumor cells were quantified in each measurement, and more than one measurement was required to confirm the diagnosis. Consequent slides with H&E staining were then reviewed to compare the histological patterns to the staining patterns in the ISH preparations.
The study protocol was approved by the Institutional Review Board of the hospital under the guidelines of the 1975 Declaration of Helsinki. Written informed consents were obtained from patients.
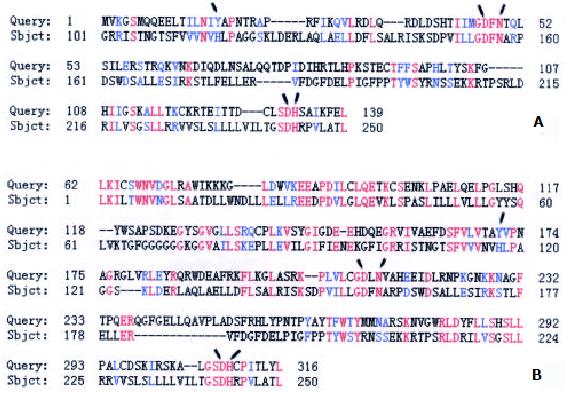
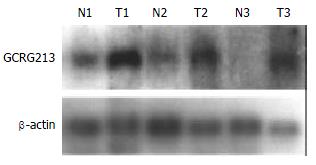
One differentially expressed cDNA band named W2 was found to be more abundant in the tumor and paratumor samples in all tested patients. W2 was sub-cloned into a pGEM-T easy vector, and confirmed by EcoR I digestion. Two subclones, GCRG213 and GCRG224, were subsequently identified[33]. Sequencing results showed that GCRG213 consisted of 1094 base pairs with an open reading frame (ORF) which encoded 142 amino acids with an estimated molecular weight of 16.4 kDa (Figure 1). This nucleotide sequence data were submitted to GenBank with accession No. AY053451. BLASTN analysis revealed that GCRG213 shared 88% homology with human retrotransposable LINE-1 element LRE2. Through conserved domain database search in GenBank, a putative conserved domain, apurinic/apyrimidinic endonucleas1 (APE), was detected in the deduced amino acid sequence of GCRG213-ORF, it shared 61.0% alignment with the C-terminal region of APE conserved domain (Figure 2). Northern blot analysis showed that GCRG213 was over-expressed in tumor tissues than in their non-tumor counterparts (Figure 3).
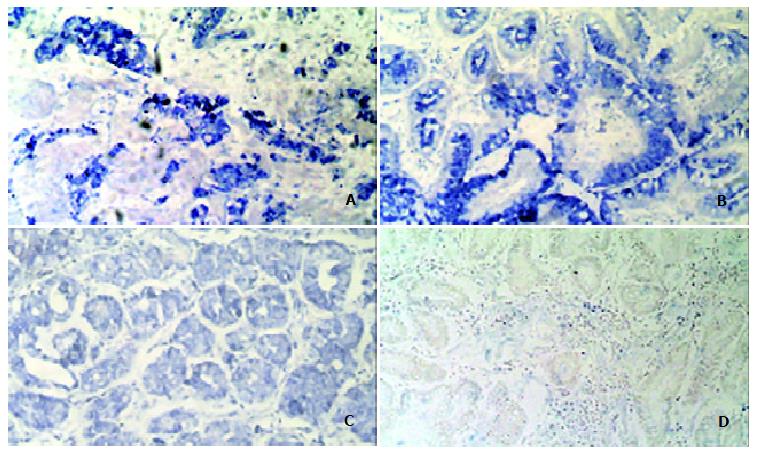
GCRG213 expression was analyzed at the mRNA level using in situ hybridization. The hybridization signal appeared as a blue color in cytoplasm.
Both the early and advanced gastric cancer tissues were stained grade 2+ ~ 3+. Eight of the 17 cases of advanced adenocarcinoma showed an invasion of cancer into the muscle layer, with all eight cases showing grade 3+ staining in the invading tumor cells (Figure 4A). Dysplasia tissues at the para-tumor region were found in 22 patients. 15/22 dysplasia tissues showed grade 2+ ~ 3+ staining of GCRG213 expression while the rest showed grade 1+ staining. Nineteen patients had intestinal metaplasia (IM) epithelia at the para-tumor tissue. 9/19 IM showed grade 2+ ~ 3+ staining, the rest showed non-staining to grade 1+ staining (Figure 4B). All normal gastric glands showed grade ± ~ + staining (Figure 4C).
In the present study, we used differential display to study the gene expression profile of human gastric cancer. One cDNA fragment, named by us as gastric cancer related gene 213 (GCRG213), was up-regulated in the gastric adenocarcinoma tissues of all 7 tested patients. Northern blot analysis confirmed the differential expression of GCRG213. As for the consistent up-regulation in gastric cancer tissues, further studies are necessary to confirm the role of GCRG213 and its expression pattern in tumors.
The nucleotide sequence of GCRG213 shared 88% homology with human retrotransposable LINE1 element LRE2. LINE-1 elements are very ancient, they constitute 20% or more of some mammalian genomes and presumably play a role in the evolution, structure, and function of mammalian genomes. LINE-1 elements contain regulatory signals and encode two proteins: one is an RNA-binding protein and the other is an APE-like enzyme which has both endonuclease and reverse transcriptase activities[38,39]. BLASTP analysis in this study showed that the deduced amino acid sequence of GCRG213-ORF shared 84% homology with the N terminal of LINE-1 ORF2 where the repair nuclease domain of APE exists.
We proposed that GCRG213-ORF be a new APE-like protein based on the following facts. First, APE conserved domain could be detected in GCRG213-ORF and the latter shared homology with the C terminal of human APE protein which is of particular relevance to the endonuclease function of APE. Furthermore, GCRG213-ORF also contains residues proposed to be important to the endonuclease activity of APE (Y171, D210, N212, D308 and H309)[40-42].
AP endonucleases have been divided into two families based on their amino acid sequence identity to either exonuclease III or Endo IV. Typically, the exonuclease III family of endonucleases accounts for approximately 95% of the repair activity in the organism. In mammals, the predominant AP endonuclease is APE (also called HAP1 or APEX), an enzyme that belongs to the ExoIII family[42]. Human APE plays an important role in the base excision repair machinery of eukaryotic cells[40]. The DNA repair activity of APE resides in the C-terminal region[41].
Many mechanisms such as hydrolysis of purine or pyrimidine, ionizing radiation, UV irradiation, and N-glycosylases may act on endogenous apurinic/apyrimidinic sites to modify DNA bases[40-43]. Unrepaired apurinic/apyrimidinic sites result in mutations during DNA replication. Apart from its DNA repair function, APE exhibits as Redox-factor-1 which is important for the activation of transcription factors, such as activator protein 1, p53 and nuclear factor kappa B. APE also regulates the transactivation and pro-apoptotic functions of p53[41]. Therefore, a role of APE in human tumorigenesis has been suggested.
APE protein is expressed in a wide range of human cells. Through immunohistochemistry detection, APE can be mainly localized in nucleus or cytoplasm or both[44] depending on the cell type. Elevated expression of APE has been reported in a number of tumors such as prostate, ovarian, cervical, colorectal and germ cell tumors, malignant gliomas, whereas the cellular localization (nuclear/cytoplasmic ratio) differs in some neoplasia (colorectal carcinomas, epithelial ovarian cancers, primary breast carcinomas and thyroid carcinomas)[45-54]. In breast cancer, APE protein expression correlates with lymph node status and angiogenesis[51] while in head-and-neck cancer, nuclear expression of APE is associated with its resistance to chemoradiotherapy and poor outcome[55]. In this study, GCRG213 overexpressed in gastric intestinal metaplasia and dysplasia of the stomach as well as early and advanced gastric adenocarcinoma. The patterns of GCRG213 expression in cancerous tissue of the early gastric cancer did not differ significantly from those in the advanced gastric carcinoma. Thus, the GCRG213 expression appears "early" in the stage of gastric adenocarcinoma. This expression pattern is consistent with that of APE in cervical, prostate, colorectal cancer and their premalignant leisions[45-47] reported.
There are two immediate implications of these findings of elevated GCRG213 expression in cancers. First, if the expression of GCRG213 could be modulated downward to, or below normal levels in the cancer cells, there may be an effect on the progression of the cancer or, the cells may become more sensitive to chemotherapeutic treatment. The latter presumes that the increase in AP endonuclease activity result in increased DNA repair activity, protecting more cancer cells against base damage than normal cells.
In conclusion, a gene, GCRG213, overexpressed in tumors was identified in this study. Because of the similarity of the expression pattern in tumors between APE and GCRG213, as well as the 61% alignment between the amino acid sequences of GCRG213-ORF and APE conserved domain, it is likely that GCRG213-ORF is a new member of the APE family. A greater understanding of alterations in the function of GCRG213 in human cancers may explore its epidemiological and therapeutic significance.
The authors would like to thank Dr. Sien-Sing Yang, the Cathey General Hospital, Taipei, Taiwan and Timothy K Lee, Ph.D., FDA, U.S.A. for their comments.
Edited by Zhao P
| 1. | González CA, Sala N, Capellá G. Genetic susceptibility and gastric cancer risk. Int J Cancer. 2002;100:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | El-Rifai W, Powell SM. Molecular biology of gastric cancer. Semin Radiat Oncol. 2002;12:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer. 2002;98:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Yoo J, Park SY, Robinson RA, Kang SJ, Ahn WS, Kang CS. ras Gene mutations and expression of Ras signal transduction mediators in gastric adenocarcinomas. Arch Pathol Lab Med. 2002;126:1096-1100. [PubMed] |
| 6. | Chang MS, Kim HS, Kim CW, Kim YI, Lan Lee B, Kim WH. Epstein-Barr virus, p53 protein, and microsatellite instability in the adenoma-carcinoma sequence of the stomach. Hum Pathol. 2002;33:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Gürel S, Dolar E, Yerci O, Samli B, Oztürk H, Nak SG, Gülten M, Memik F. Expression of p53 protein and prognosis in gastric carcinoma. J Int Med Res. 1999;27:85-89. [PubMed] |
| 8. | Liu XP, Tsushimi K, Tsushimi M, Kawauchi S, Oga A, Furuya T, Sasaki K. Expression of p21(WAF1/CIP1) and p53 proteins in gastric carcinoma: its relationships with cell proliferation activity and prognosis. Cancer Lett. 2001;170:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Sato K, Tamura G, Tsuchiya T, Endoh Y, Usuba O, Kimura W, Motoyama T. Frequent loss of expression without sequence mutations of the DCC gene in primary gastric cancer. Br J Cancer. 2001;85:199-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Lee JH, Abraham SC, Kim HS, Nam JH, Choi C, Lee MC, Park CS, Juhng SW, Rashid A, Hamilton SR. Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol. 2002;161:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Zhou Y, Gao SS, Li YX, Fan ZM, Zhao X, Qi YJ, Wei JP, Zou JX, Liu G, Jiao LH. Tumor suppressor gene p16 and Rb expression in gastric cardia precancerous lesions from subjects at a high incidence area in northern China. World J Gastroenterol. 2002;8:423-425. [PubMed] |
| 12. | Waki T, Tamura G, Tsuchiya T, Sato K, Nishizuka S, Motoyama T. Promoter methylation status of E-cadherin, hMLH1, and p16 genes in nonneoplastic gastric epithelia. Am J Pathol. 2002;161:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Hsieh HF, Yu JC, Ho LI, Chiu SC, Harn HJ. Molecular studies into the role of CD44 variants in metastasis in gastric cancer. Mol Pathol. 1999;52:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Kikuchi T, Itoh F, Toyota M, Suzuki H, Yamamoto H, Fujita M, Hosokawa M, Imai K. Aberrant methylation and histone deacetylation of cyclooxygenase 2 in gastric cancer. Int J Cancer. 2002;97:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Krieg A, Mahotka C, Krieg T, Grabsch H, Müller W, Takeno S, Suschek CV, Heydthausen M, Gabbert HE, Gerharz CD. Expression of different survivin variants in gastric carcinomas: first clues to a role of survivin-2B in tumour progression. Br J Cancer. 2002;86:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Yu J, Leung WK, Ebert MP, Ng EK, Go MY, Wang HB, Chung SC, Malfertheiner P, Sung JJ. Increased expression of survivin in gastric cancer patients and in first degree relatives. Br J Cancer. 2002;87:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ebert MP, Günther T, Hoffmann J, Yu J, Miehlke S, Schulz HU, Roessner A, Korc M, Malfertheiner P. Expression of metallothionein II in intestinal metaplasia, dysplasia, and gastric cancer. Cancer Res. 2000;60:1995-2001. [PubMed] |
| 19. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 835] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 20. | Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, Kenji Yagi O, Saitoh K, Takeshita K, Iwai T. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Saitoh T, Mine T, Katoh M. Up-regulation of WNT8B mRNA in human gastric cancer. Int J Oncol. 2002;20:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Li Zy, Wang Yj, Song Jp, Kataoka H, Yoshii S, Gao Cm, Wang Yp, Zhou Jn, Ota S, Tanaka M. Genomic structure of the human beta-PIX gene and its alteration in gastric cancer. Cancer Lett. 2002;177:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Wang G, Wang M, You W, Li H. [Cloning and primary expression analyses of down-regulated cDNA fragment in human gastric cancer]. Zhonghua Yixue Yichuanzue Zazhi. 2001;18:43-47. [PubMed] |
| 25. | Wang JH, Chen SS. Screening and identification of gastric adenocarcinoma metastasis-related genes by using cDNA microarray coupled to FDD-PCR. Shengwu Huaxue Yu Shengwu Wui Xuebao (Shanghai). 2002;34:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54-59. [PubMed] |
| 27. | Saitoh T, Mine T, Katoh M. Molecular cloning and expression of proto-oncogene FRAT1 in human cancer. Int J Oncol. 2002;20:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 28. | Linē A, Stengrēvics A, Slucka Z, Li G, Jankevics E, Rees RC. Serological identification and expression analysis of gastric cancer-associated genes. Br J Cancer. 2002;86:1824-1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Lee S, Baek M, Yang H, Bang YJ, Kim WH, Ha JH, Kim DK, Jeoung DI. Identification of genes differentially expressed between gastric cancers and normal gastric mucosa with cDNA microarrays. Cancer Lett. 2002;184:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M. Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol. 2002;8:580-585. [PubMed] |
| 31. | El-Rifai W, Smith MF, Li G, Beckler A, Carl VS, Montgomery E, Knuutila S, Moskaluk CA, Frierson HF, Powell SM. Gastric cancers overexpress DARPP-32 and a novel isoform, t-DARPP. Cancer Res. 2002;62:4061-4064. [PubMed] |
| 32. | Wang G, Wang M, Wu B, You W. [A lamin-like protein gene is down-regulated in human gastric cancer]. Zhonghua Yixue Yichuanzue Zazhi. 2003;20:119-122. [PubMed] |
| 33. | Wang GS, Wang MW, Wu BY, You WD, Yang XY. A novel gene, GCRG224, is differentially expressed in human gastric mucosa. World J Gastroenterol. 2003;9:30-34. [PubMed] |
| 34. | Wang GS, Wang MW, You WD, Wang HF, Feng MF. [Fluorescent mRNA differential display technique]. Zhongguo Yingyong Shenglixue Zazhi. 2000;16:373-376. [PubMed] |
| 35. | Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389-3402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53569] [Cited by in RCA: 52305] [Article Influence: 1868.0] [Reference Citation Analysis (0)] |
| 36. | Komminoth P. Digoxigenin as an alternative probe labeling for in situ hybridization. Diagn Mol Pathol. 1992;1:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Komminoth P, Merk FB, Leav I, Wolfe HJ, Roth J. Comparison of 35S- and digoxigenin-labeled RNA and oligonucleotide probes for in situ hybridization. Expression of mRNA of the seminal vesicle secretion protein II and androgen receptor genes in the rat prostate. Histochemistry. 1992;98:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Furano AV. The biological properties and evolutionary dynamics of mammalian LINE-1 retrotransposons. Prog Nucleic Acid Res Mol Biol. 2000;64:255-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Volff JN, Körting C, Froschauer A, Sweeney K, Schartl M. Non-LTR retrotransposons encoding a restriction enzyme-like endonuclease in vertebrates. J Mol Evol. 2001;52:351-360. [PubMed] |
| 40. | Fritz G. Human APE/Ref-1 protein. Int J Biochem Cell Biol. 2000;32:925-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 426] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Wilson DM, Barsky D. The major human abasic endonuclease: formation, consequences and repair of abasic lesions in DNA. Mutat Res. 2001;485:283-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 312] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Meira LB, Devaraj S, Kisby GE, Burns DK, Daniel RL, Hammer RE, Grundy S, Jialal I, Friedberg EC. Heterozygosity for the mouse Apex gene results in phenotypes associated with oxidative stress. Cancer Res. 2001;61:5552-5557. [PubMed] |
| 44. | Duguid JR, Eble JN, Wilson TM, Kelley MR. Differential cellular and subcellular expression of the human multifunctional apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme. Cancer Res. 1995;55:6097-6102. [PubMed] |
| 45. | Kakolyris S, Kaklamanis L, Engels K, Turley H, Hickson ID, Gatter KC, Harris AL. Human apurinic endonuclease 1 expression in a colorectal adenoma-carcinoma sequence. Cancer Res. 1997;57:1794-1797. [PubMed] |
| 46. | Kelley MR, Cheng L, Foster R, Tritt R, Jiang J, Broshears J, Koch M. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin Cancer Res. 2001;7:824-830. [PubMed] |
| 47. | Xu Y, Moore DH, Broshears J, Liu L, Wilson TM, Kelley MR. The apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme is elevated in premalignant and malignant cervical cancer. Anticancer Res. 1997;17:3713-3719. [PubMed] |
| 48. | Schindl M, Oberhuber G, Pichlbauer EG, Obermair A, Birner P, Kelley MR. DNA repair-redox enzyme apurinic endonuclease in cervical cancer: evaluation of redox control of HIF-1alpha and prognostic significance. Int J Oncol. 2001;19:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Herring CJ, West CM, Wilks DP, Davidson SE, Hunter RD, Berry P, Forster G, MacKinnon J, Rafferty JA, Elder RH. Levels of the DNA repair enzyme human apurinic/apyrimidinic endonuclease (APE1, APEX, Ref-1) are associated with the intrinsic radiosensitivity of cervical cancers. Br J Cancer. 1998;78:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Moore DH, Michael H, Tritt R, Parsons SH, Kelley MR. Alterations in the expression of the DNA repair/redox enzyme APE/ref-1 in epithelial ovarian cancers. Clin Cancer Res. 2000;6:602-609. [PubMed] |
| 51. | Kakolyris S, Kaklamanis L, Engels K, Fox SB, Taylor M, Hickson ID, Gatter KC, Harris AL. Human AP endonuclease 1 (HAP1) protein expression in breast cancer correlates with lymph node status and angiogenesis. Br J Cancer. 1998;77:1169-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Robertson KA, Bullock HA, Xu Y, Tritt R, Zimmerman E, Ulbright TM, Foster RS, Einhorn LH, Kelley MR. Altered expression of Ape1/ref-1 in germ cell tumors and overexpression in NT2 cells confers resistance to bleomycin and radiation. Cancer Res. 2001;61:2220-2225. [PubMed] |
| 53. | Bobola MS, Blank A, Berger MS, Stevens BA, Silber JR. Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clin Cancer Res. 2001;7:3510-3518. [PubMed] |
| 54. | Russo D, Arturi F, Bulotta S, Pellizzari L, Filetti S, Manzini G, Damante G, Tell G. ApeI/Ref-I expression and cellular localization in human thyroid carcinoma cell lines. J Endocrinol Invest. 2001;24:RC10-RC12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Koukourakis MI, Giatromanolaki A, Kakolyris S, Sivridis E, Georgoulias V, Funtzilas G, Hickson ID, Gatter KC, Harris AL. Nuclear expression of human apurinic/apyrimidinic endonuclease (HAP1/Ref-1) in head-and-neck cancer is associated with resistance to chemoradiotherapy and poor outcome. Int J Radiat Oncol Biol Phys. 2001;50:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |