Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1179
Revised: March 1, 2003
Accepted: March 5, 2003
Published online: June 15, 2003
AIM: To study the relationship between the expression profiles of a plant-associated human cancer antigen and carcinogenesis of esophagus and its significance.
METHODS: We analyzed expression of a plant-associated human cancer antigen in biopsy specimens of normal (n = 29), mildly hyperplastic (n = 29), mildly (n = 30), moderately (n = 27) and severely dysplastic (n = 29) and malignant esophageal (n = 30) tissues by immunohistochemistry.
RESULTS: The plant-associated human cancer antigen was mainly confined to the cytoplasm and showed diffuse type of staining. Positive staining was absent or weak in normal (0/30) and mildly hyperplastic tissue samples (2/29), while strong staining was observed in severe dysplasia (23/29) and carcinoma in situ (24/30). There was significant difference of its expression between normal mucosa and severely dysplastic tissues (P < 0.001) or carcinoma in situ (P < 0.001). Significant difference was also observed between mild dysplasia and severe dysplasia (P < 0.001) or carcinoma in situ (P < 0.001). An overall trend toward increased staining intensity with increasing grade of dysplasia was found. There was a linear correlation between grade of lesions and staining intensity (r = 0.794, P < 0.001). Samples from esophageal cancer showed no higher levels of expression than those in severely dysplastic lesions (P > 0.05).
CONCLUSION: The abnormal expression of this plant-associated human cancer antigen in esophageal lesions is a frequent and early finding in the normal-dysplasia-carcinoma sequence in esophageal carcinogenesis. It might contribute to the carcinogenesis of esophageal cancer. The abnormal expression of this plant-associated human cancer antigen in esophageal lesion tissues may serve as a potential new biomarker for early identification of esophageal cancer.
- Citation: Fu J, Qu P, Li M, Tian HM, Zheng ZH, Zheng XW, Zhang W. Expression of a plant-associated human cancer antigen in normal, premalignant and malignant esophageal tissues. World J Gastroenterol 2003; 9(6): 1179-1181
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1179.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1179
Despite three decades of progress in cancer treatment, esophageal cancer remains a significant health problem worldwide with a very low 5-year survival rate and a rapid increase in its incidence[1-4]. Esophageal squamous cell carcinoma is believed to develop progressively through a dysplasia-carcinoma sequence[5,6]. The evolution of sequential histological changes from normal to dysplasia and carcinoma in situ suggests that specific biomarkers may be expressed at different points in the evolution of esophageal carcinoma[7]. Therefore, research directed toward the discovery of new biomarkers that can aid in the early detection of esophageal neoplasm has been intensified in recent years.
In the 1990s, Dr. Zhen-Hai Zheng separated and identified a glycoprotein (molecular weight: 46 kilo-dalton) from a lower plant. Early experiments demonstrated its stimulatory activity on the growth of esophageal cancer cells. Recently the differential stimulatory effects of this plant antigen on isolated lymphocytes from patients with dysplasia or breast cancer have been confirmed. Their experiments also suggested that this plant antigen might have an associated counterpart cancer antigen in human.
In this research, we aimed to study the relationship between expression profiles of this human cancer antigen and carcinogenesis of esophagus and its significance.
Biopsy specimens were obtained from the Cancer Hospital (Chinese Academy of Medical Sciences) in Beijing. These samples were taken from patients with morphologically normal (n = 29), mildly hyperplastic (n = 29) esophageal mucosa, mildly (n = 30), moderately (n = 27) or severely (n = 29) dysplastic lesions, or squamous carcinomas in situ (n = 30). All samples were routinely fixed in 10% buffered formalin, embedded in paraffin, and cut into 4 μm sections. Samples were selected based on the pathological diagnosis and reviewed by a pathologist to ensure the correct diagnosis.
SP-ABC kit was purchased from Beijing Zhong Shan Biotechnology Co. Ltd. Rabbit polyclonal antibody anti-plant antigen was kindly provided by professor Zhen-Hai Zheng, diluted to 2 × 10-2 g•l-1 before use.
The immunohistochemical localization of the plant-associated human cancer antigen was performed using a modified ABC technique[8]. Briefly, tissue sections were deparaffinized in xylene and rehydrated in a series of ethanol solutions (100%-50%). The sections were then microwaved for 15 min to restore antigens in 0.01M citric acid solutions. The endogenous peroxidase activity was blocked by incubation in a 3% hydrogen peroxide solution for 20 min at 23 °C. This was followed by preincubation with 1.5% normal sheep serum to minimize nonspecific binding of the second antibody. The sections were incubated at 4 °C overnight with rabbit polyclonal antibody anti-plant protein. After being washed three times in PBS, the sections were incubated with biotinylated sheep anti-rabbit IgG for 20 min at 37 °C and were washed three times in PBS. Sections were then incubated with streptavidin-peroxidase for 20 min at 37 °C, followed by 2 min staining with DAB-H2O2 solution and nuclear counterstained with hematoxylin. For negative controls, the rabbit anti-plant protein polyclonal antibody was replaced by normal rabbit serum.
The stained sections were reviewed and scored independently by two investigators using an Olympus microscope. The intensity of staining was graded on a scale of 0 to 3 as follows: 0 meaning the amounts of positive cells were less than 10%; 1, weak positive, the amounts of positive cells were more than 10%, but less than 30%; 2, positive, the amounts of positive cells were more than 30% but less than 50%; and 3, strong positive, the amounts of positive cells were more than 50%[9]. The grading for each section used for data analysis was the highest intensity seen on the section.
The mean values of the staining intensity for different grades of diseases were compared by analysis of variance. Linear regression analysis was done to evaluate the linear correlation between lesion severity and staining intensity. Statistical analysis was made using Fisher's exact test to determine the association between normal or dysplastic tissues and tumors. P values were generated using SPSS 10.0 for Windows. Statistical significance was accepted at the P < 0.05 level.
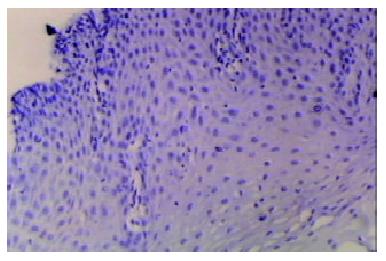
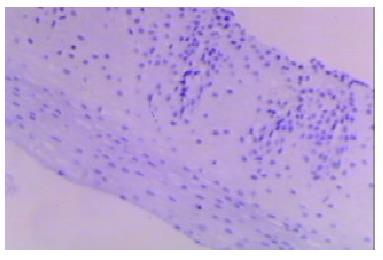
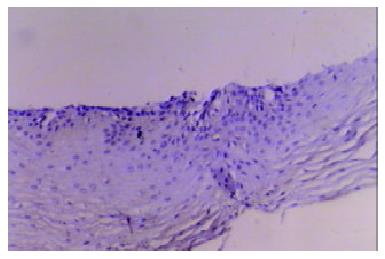
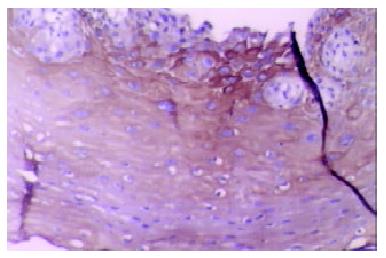
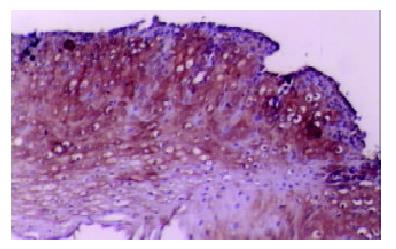
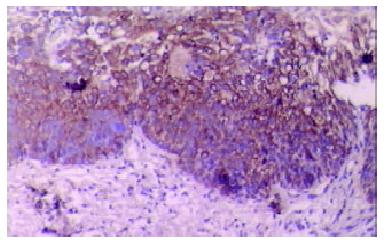
Our finding suggested that the plant-associated human cancer antigen was mainly confined to the cytoplasma and showed diffuse type of staining, enormous nuclear staining could also be observed in severe dysplastic and carcinoma in situ tissue samples. Only background staining was observed in basal layers underlying epithelium. Staining was absent to weak in normal and mildly hyperplastic tissue samples (Figure 1 Figure 2, Figure 3, Figure 4, Figure 5, Figure 6). Positive staining was absent to weak in normal (0/30) and mildly hyperplastic tissue samples (2/29), while high positive rate was observed in severe dysplasia (23/29) and carcinoma in situ (24/30).
There was significant difference of its expression between normal mucosa and severely dysplastic tissues (P < 0.001) or esophageal cancer (P < 0.001). Significant difference was also observed between mild dysplasia and severe dysplasia (P < 0.001) or esophageal cancer (P < 0.001) (Table 1). Samples from esophageal cancer showed no higher levels of its expression than seen in severely dysplastic lesions (P > 0.05). An overall trend toward increased staining intensity with increasing grade of dysplasia was found. There was a linear correlation between grade of lesion and staining intensity (r = 0.794, P < 0.001) (Table 2).
| Esophageal tissues | Total cases | Positive staining | Negative staining | %Positive |
| Normal | 29 | 0 | 18 | 0 |
| Hyperplasia | 29 | 2 | 27 | 6.9 |
| Dysplasia | ||||
| Mild | 30 | 8 | 22 | 26.7 |
| Moderate | 27 | 17 | 10 | 63.0 |
| Severe | 29 | 23 | 6 | 79.3 |
| aCIN | 30 | 24 | 6 | 80.0 |
| Histologic diagnisis | Total cases | Staining intensitya | Biopsies staining with intensity > 2 (%) |
| Normal | 29 | 0 | 0 |
| Dysplasia | |||
| Mild | 30 | 0.270.52 | 3.3 |
| Moderate | 27 | 1.000.93 | 25.9 |
| Severe | 29 | 1.380.96 | 41.4 |
| CIN | 30 | 1.330.92 | 43.3 |
We have evaluated esophageal mucosal expression of the plant-associated human cancer antigen in a variety of normal, precancerous or malignant esophageal tissue specimens. Esophageal biopsy specimens with normal histology or low-grade dysplasia did not express significant levels of this human cancer antigen. Its intensive expression was observed in high-grade dysplastic and malignant lesions. There was an overall trend toward increased staining intensity with increasing grade of dysplasia, and the largest difference was observed between mild and severe dysplastic lesions, suggesting that its up-regulation of expression may be involved in esophageal carcinogenesis. Since esophageal carcinogenesis is a multi-stage process, early detection is very crucial[10,11]. This human cancer antigen might be a useful diagnostic marker for advanced dysplasia and could serve as a useful target for biological treatment and for bio-prevention in high-risk patients diagnosed having dysplasia or esophageal carcinoma.
In this report, we also studied the expression distribution of this human cancer antigen in abnormal human sections. Although this antigen was mainly expressed in cytoplasma, enormous nuclear staining could also be observed in severely dysplastic and malignant tissue samples. Combined with previous conclusions, our finding suggested that this protein might be involved in signal transduction pathway or cell cycle control. Its intracellular translocation from cytoplasma to nuclei might play an important role in the transition from low-grade dysplasia to severe lesions and neoplasm. The mechanism of action of this cancer antigen in esophageal carcinogenesis still awaits further studies.
The ability to identify the plant-associated human cancer antigen in esophageal biopsy specimens from patients with dysplasia may provide a useful method for early detection, identification of high-risk patients, monitoring response to therapy. Further studies are needed to separate and identify this protein and its encoding gene in order to fully understand the regulatory mechanism underlying its aberrant expression and its role in esophageal carcinogenesis.
Edited by Ma JY
| 1. | McCann J. Esophageal cancers: changing character, increasing incidence. J Natl Cancer Inst. 1999;91:497-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Flood WA, Forastiere AA. Esophageal cancer. Curr Opin Oncol. 1995;7:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Flood WA, Forastiere AA. Esophageal cancer. Cancer Treat Res. 1998;98:1-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 4. | Blot WJ. Esophageal cancer trends and risk factors. Semin Oncol. 1994;21:403-410. [PubMed] |
| 5. | Liu FS, Wang QL, Goldman H. Pathology of the gastrointestinal tract. Philadelphia: Saunders 1992; 439-458. |
| 6. | Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 441] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Stemmermann G, Heffelfinger SC, Noffsinger A, Hui YZ, Miller MA, Fenoglio-Preiser CM. The molecular biology of esophageal and gastric cancer and their precursors: oncogenes, tumor suppressor genes, and growth factors. Hum Pathol. 1994;25:968-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Zhang W, Rashid A, Wu H, Xu XC. Differential expression of retinoic acid receptors and p53 protein in normal, premalignant, and malignant esophageal tissues. J Cancer Res Clin Oncol. 2001;127:237-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Xu M, Jin YL, Fu J, Huang H, Chen SZ, Qu P, Tian HM, Liu ZY, Zhang W. The abnormal expression of retinoic acid receptor-beta, p 53 and Ki67 protein in normal, premalignant and malignant esophageal tissues. World J Gastroenterol. 2002;8:200-202. [PubMed] |
| 10. | Kelloff GJ, Boone CW, Steele VK, Perloff M, Crowell J, Doody LA. Development of chemopreventive agents for lung and upper aerodigestive tract cancers. J Cell Biochem Suppl. 1993;17F:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Natl Cancer Inst. 1998;90:1514-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |