Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1165
Revised: February 1, 2003
Accepted: February 17, 2003
Published online: June 15, 2003
Information about the mechanisms that generate mutations in eukaryotes is likely to be useful for understanding human health concerns, such as genotoxicity and cancer. Eukaryotic mutagenesis is largely the outcome of attacks by endogenous and environmental agents. Except for DNA repair, cell cycle checkpoints and DNA damage avoidance, cells have also evolved DNA damage tolerance mechanism, by which lesion-targeted mutation might occur in the genome during replication by specific DNA polymerases to bypass the lesions (translesion DNA synthesis, TLS), or mutation on undamaged DNA templates (untargeted mutation) might be induced. DNA polymerase ζ (pol ζ), which was found firstly in budding yeast Saccharomyces cerevisiae and consists of catalytic subunit scRev3 and stimulating subunit scRev7, has received more attention in recent years. Pol ζ is a member of DNA polymerase δ subfamily, which belongs to DNA polymerase B family, and exists in almost all eukaryotes. Human homolog of the scRev3 gene is located in chromosome region 6q21, and the mouse equivalent maps to chromosome 10, distal to the c-myb gene and close to the Macs gene. Alternative splicing, upstream out-of frame ATG can be found in yeast scRev3, mouse and human homologs. Furthermore, the sequence from 253-323 immediate upstream of the AUG initiator codon has the potential to form a stem-loop hairpin secondary structure in REV3 mRNA, suggesting that human REV3 protein may be expressed at low levels in human cells under normal growth conditions. The functional domain analysis showed that yeast Rev3-980 tyrosine in conserved region II is at the polymerase active site. Human REV3 amino acid residues 1776-2195 provide a REV7 binding domain, and REV7 amino acid residues 1-211 provide a bind domain for REV1, REV3 and REV7 itself. More interestingly, REV7 interacts with hMAD2 and therefore might function in the cell cycle control by affecting the activation of APC (anaphase promoting complex). Currently it has been known that pol ζ is involved in most spontaneous mutation, lesion-targeted mutation via TLS, chemical carcinogen induced untargeted mutation and somatic hypermutation of antibody genes in mammalian. In TLS pathway, pol ζ acts as a "mismatch extender" with combination of other DNA polymerases, such as pol ι. Unlike in yeast, it was found that pol ζ also functioned in mouse embryonic development more recently. It was hypothesized that the roles of pol ζ in TLS and cell cycle control might contribute to mouse embryonic lethality.
- Citation: Zhu F, Zhang M. DNA polymerase ζ: new insight into eukaryotic mutagenesis and mammalian embryonic development. World J Gastroenterol 2003; 9(6): 1165-1169
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1165
The increase of environmental cancer has been received intensive attention in recent years[1-6]. An arresting example is that tobacco smoke significantly increases the risks for oral[7,8], esophageal[3,6,9,10], bladder[11-14], pancreas[13], gastric[15] and colorectal cancers[16]. To understand the relationship between environmental agents and cancer is a noteworthy hotspot, by which it is possible to establish a system to prevent and control environmental cancers.
Information about the mechanisms that generate mutations in eukaryotes is likely to be useful for understanding human health concerns, such as genotoxicity and cancer. Eukaryotic mutagenesis is largely the outcome of attacks by endogenous and environmental agents. However, the cells have evolved sophisticated systems in response to DNA damage, including DNA repair and cell cycle checkpoints. Even when DNA repair systems and cell cycle checkpoints are fully functional, some damage can still persist in the genome during replication under circumstances such as: (i) when cells sustain significant DNA damage; (ii) when a particular damage is poorly repaired; or (iii) when some genomic regions are inefficiently repaired. DNA damage frequently blocks replication. Such blockage can be overcome by error-free or error-prone translesion DNA synthesis (TLS) bypass, employing specialized DNA polymerases and proteins for promoting continuous nascent strand extension at forks blocked by the presence of unrepaired DNA damage at the cost of increasing mutation frequency[17]. Alternatively, mutation can be avoided by DNA damage avoidance[18], or occur on undamaged DNA template and lead to untargeted mutation via damage tolerance[19].
DNA polymerase ζ (pol ζ), consisting of catalytic subunit scRev3 and stimulating scRev7 in budding yeast Saccharomyces cerevisiae, has received more attention in recent years. It is thought to be the major component of error-prone TLS pathway[20-24], although a number of other polymerases might be involved in this process[25]. In Saccharomyces cerevisiae, TLS pathway pasting many types of DNA damage in yeast depends on the activities of pol ζ and Rev1p, which is a major source of DNA-damage-induced substitutions and frameshifts and of spontaneous mutations[21,22,26-30]. It has been speculated and demonstrated later that human pol ζ plays a major role in UV-induced mutagenesis and somatic hypermutation in antibody genes[31-34]. More recently, it was found that human pol ζ was also involved in mammalian untargeted mutagenesis, and the expression of human mutator REV3 could be upregulated at transcriptional level in response to chemical carcinogen N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)[35], which could induce gastric cancer[36-39] and colorectal cancer[40,41]. Evidences also suggested that pol ζ was concerned with cell cycle control and early embryonic development[42-45]. The aim of this paper was to review the structural and functional features of pol ζ, and its roles in mutagenesis and embryonic development as well.
In the budding yeast Saccharomyces cerevisiae, the scRev3-scRev7 complex is the sixth eukaryotic DNA polymerase to be described, and is therefore called DNA polymerase ζ [22]. The catalytic subunit Rev3 is a member of family B DNA polymerases, which contains six conserved motifs[46,47]. Mutation analysis in vivo and the X-ray crystal structures of family B polymerases reveal that yeast scRev3-980 tyrosine in conserved region II is at the polymerase active site[48]. Investigation suggests that homologues of the yeast scRev3 gene are found in almost all eukaryotes, including Arabidopsis thaliana, Drosophila melanogaster, Schizosaccharomyces pombe, mouse and specifically humans[30,31,49-53].
Human homolog of the Saccharomyces cerevisiae scRev3 gene is located on chromosome region 6q21, and the mouse equivalent maps to chromosome 10, distal to the c-myb gene, and close to the Macs gene[50,52]. The full-length cDNA of human REV3 consists of 10919 nucleotides, with a putative open reading frame of 9390 bp[31,50,51]. Human REV3 gene contains 33 exons in about 200 kb of genomic DNA, in which an additional exon, alternative splicing event and an upstream out-of frame ATG have been demonstrated[31,50]. The same alternative splicing has also been observed in mouse, with a 128 bp exon inserted between nt +139 and +140 in approximately 50% of the transcripts[52]. An upstream out-of-frame ATG with an ORF that terminates within the main ORF is also found in the yeast gene[21], suggesting that it may be evolutionally conserved in all pol ζ genes. The sequence context of the upstream gene performs a similar function to that of its yeast counterpart. Interestingly, three stretches of sequences, GGCAGTGGCGGC, AGGGGAGGGGGC, and GCCGCCGCCGCTGC, are duplicated in the 5 untranslated region constituting 323 nucleotides. Furthermore, the sequence from 253-323 immediate upstream of the AUG initiator codon has the potential to form a stem-loop hairpin secondary structure in REV3 mRNA[51]. Such primary structural features and the secondary structure in the 5' untranslated region are expected to reduce the translational efficiency of the message, suggesting that human REV3 protein may be expressed at low levels in human cells under normal growth conditions.
The predicted homogous proteins in human and mouse are a little over twice the length of the yeast scReve protein (1504 residues), i.e., 3130 amino acids with an expected mass of 353 kDa and a calculated pi of 8.7 in human, and 3122 amino acids in mouse respectively. The homologous proteins of yeast scRev3 are highly conserved[31,51,52]: (i) in the N-terminal part, the overall homology between yeast scRev3/Drosophila DmRev3, yeast scRev3/human REV3 and Drosophila DmRev3/human REV3 amounts to respectively 33.3%, 35.0% and 50.5% identical amino acids. (ii) in a region of 850 residues at the carboxyl terminus, the overall homology between yeast scRev3/human REV3 amounts to 39% identical amino acids, and (iii) in a 55-residue region in the middle of both scRev3 and REV3 proteins, with 29% identity. But little similarity can be found in the intervening regions[31].
The carboxyl terminus region of yeast Rev3 homologue proteins in human, mouse and Drosophila contains the six conserved sequence motifs characterized by DNA polymerases in the right order, including the canonical hexapeptide motifs within regions 1 and 2, YGDTDS and SLYPSI, which are found jointly only in type B DNA polymerases[46,47]. Further alignment shows that pol ζ is a member of DNA polymerase δ family with two specifically conserved motifs ζ I and ζ II in pol ζ, and in the N-terminal part, a conserved glycine repeat motif (G-x4-G-x2-G-x8-G-x3-YFY) in pol δ is also present in the homologues which have been implicated in nucleotide binding[51]. Outside the six DNA polymerase motifs in the C-terminal, both yeast scRev3 and human REV3 proteins contain a putative zinc finger DNA binding region, and the location of this putative zinc finger is also highly conserved from yeast to humans[51]. Such structural features are consistent with the notion that the C-terminal region of scRev3 homologue serves as the catalytic domain during nucleotide polymerization, while its N-terminal region may provide sites for protein-protein interactions with other factors such as a putative yeast scRev7 homologue during translesional DNA synthesis.
However, the existence of a much larger nonhomologous or species-specific region in the expected human protein suggests that pol ζ may perform a wider range of functions in the higher eukaryotes. In Saccharomyces cerevisiae, it was found that LexA-scRev3 and Gal4-scRev7 fusions interacted with each other[22]. More recently, studies showed that human REV3 amino acid residues 1776-2195 provided a REV7 binding domain, and REV7 amino acid residues 1-211 provided a bind domain for REV1, REV3 and REV7 itself [54,55]. REV7, the stimulating subunit of pol ζ which is located on chromosome 1p36, displays 23% identity and 53% similarity with scRev7, as well as 23% identity and 54% similarity with the human mitotic checkpoint protein hMAD2. And yeast two-hybrid assay suggests that REV7 may interact with hMAD2 but not with hMAD1[54]. It is possible that REV3, REV7, and hMAD2 might be capable of forming a stable triprotein complex.
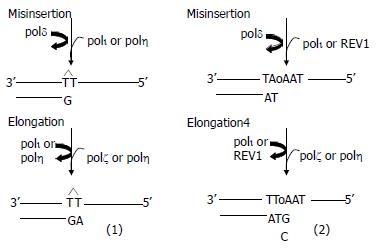
DNA damage induced elevation of mutations during the course of translesion replication is likely to be an important contributory cause in the development of many cancers[27]. With in vitro and in vivo investigation, it has been clear that pol ζ plays a role in an error-prone way. Yeast pol ζ can bypass T-T cyclobutane dimer, but not (6-4) T-T photoproduct and abasic site, inserting an incorrect nucleotide with relatively low efficiency in vitro (finc values range from 4.1 × 10-3 to 1.9 × 10-5)[22,56]. When combined with REV1 (transfers a dCMP residue from dCTP to the 3' end of a DNA primer in a template-dependent reaction opposite abasic site), pol ι (inserts a deoxynucleotide opposite the (6-4) T-T photoproduct and abasic site) or pol η (bypasses T-T cyclobutane dimer with relative high accuracy and efficiency), pol ζ can bypass all three types of lesions with more efficiency (fext values range from 10-1 to 10-2) at elongating from a mismatched terminus, which develops the TLS two-step model (Figure 1)[56-58].
Mutation caused by TLS is usually designated targeted mutation. However, mutation can also occur on undamaged DNA template and therefore is called untargeted mutation (UTM), which has been described in SOS-induced mutagenesis in E. coli[59]. It has been known that untargeted and targeted mutations caused by SOS response in E. coli both result from the inhibition of DNA polymerase functions that normally maintain fidelity and the involvement of DNA polymerases with low fidelity, which include DNA pol III, pol IV (dinB), pol V (UmuD'2C) and other factors (RecA*, β-sliding clamp, γ-clamp loading complex and single-stranded binding protein)[60-64]. Using mating experiments with excision deficient strains of Saccharomyces cerevisiae, Lawrence and Christensen found that up to 40% of cycl-91 revertants induced by UV were untargeted, showing that a reduction in fidelity of DNA replication[65]. In mouse T-lymphoma cells, stress response induced by DNA damage agents (8-methoxy-psoralen or UVA) leads to specific, delayed and untargeted mutations[66]. It has been found that low concentration N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), a carcinogen which can induce gastric cancer, could induce mammalian UTM[19]. However, it is not clear which factor capable of inhibiting fidelity can be induced or activated. More recently, it was found that pol ζ might be involved in the mammalian UTM induced by MNNG. The transcriptional level of REV3 gene is upregulated when human cells are treated by low concentration MNNG. Furthermore, human cells, in which the function of pol ζ is inhibited by antisense REV3 RNA, display characteristics of both anti UTM and targeted mutation[35].
Pol ζ also functions in somatic hypermutation[33,34]. Accumulation of somatic mutations in the V(H) genes of memory B cells from transgenic mice which express antisense RNA to a portion of mouse REV3 is decreased, particularly among those that generate amino acid replacements enhancing the affinity of the B cell receptor to the hapten[33]. In addition, inhibition of the mouse mRev3 by specific phosphorothioate-modified oligonucleotides impaires Ig and bcl-6 hypermutation and UV damage-induced DNA mutagenesis, without affecting cell cycle or viability[34].
In Saccharomyces cerevisiae, pol ζ is not essential for cell viability, as indicated by the fact that haploids carrying a complete deletion of Rev3 are viable[21]. Besides, human fibroblast cells expressing high levels of an REV3 antisense RNA fragment grow normally[31]. However, recent evidences have shown that pol ζ is essential for cell viability during embryonic development in mammals (Table 1)[43-45,67].
| Size | Reduced at day 10.5 |
| Viability | Usually aborted around day 12.5 |
| Inner cell mass (ICM) | Diminished expansion |
| Haematopoietic cells | No haematopoietic cells developed other than erythrocytes |
| Morphogenesis of embryo | 1. Abnormalities in the development and maintenance of embryonic mesoderm |
| 2. Predominant disorder and lack of integrity mainly in mesenchymal tissues, including heart and large blood vessels |
It has been realized that numerous DNA lesions caused by unavoidable oxidative and hydrolytic processes are constantly formed in genomes[68]. Double-strand breaks can form when DNA replication forks encounter nicked templates, and these stalled replication forks must be reactivated by replication or repair. Unlike cells in adult tissues or in culture, which have mechanisms to cease division or DNA replication temporarily in order to allow accurate and specific DNA repair enzymes to act before proceeding through the cell cycle, embryonic development adheres to a strict temporal program that requires rapid cell division. Under such conditions, enzymes that can rapidly bypass DNA lesions may be expected to be particularly important, and an intolerable load of damaged DNA in critical embryonic or extra-embryonic cells would then lead to death. On the other hand, the proliferating cells in the embryo might gradually accumulate DNA damage and ES cells may be a special case of a cell type primed to undergo apoptosis after accumulated low levels of DNA damage[69]. During the developmental stage of the mRev3-defective mouse embryos before embryonic death, the embryos are still able to propagate rapidly and differentiate through many cell divisions.
It is noteworthy that mouse mRev3 is most highly expressed in mesodermal tissues and embryonic death coincides with the period of more widely distributed mRev3l expression. High-level expression of mouse mRev3 is developmentally regulated during embryogenesis, occurring first in early somatogenesis and then in other mesodermal tissues up to at least 11.5 d post coitum[45]. This differential expression seems likely to account for the predominant disorder and lack of integrity found mainly in mesenchymal tissues. Lack of proper development of the heart and large blood vessels might in itself be the immediate cause of death. Also, as mouse mRev3 is normally expressed within extraembryonic membranes, the absence of functional mRev3 in mouse mRev3-/- embryos could be the cause of the pericardial sac edema, yolk sac fragility and weak attachment to the decidual implantation site. Yolk-sac malfunction can induce osmotic imbalance, leading to edema, whereas delayed and/or suboptimal chorioallantoic fusion can result in an implantation defect. Defects of the chorioallantoic placenta or yolk sac are a common cause of murine lethality in utero and could contribute to the embryonic lethality of mouse mRev3 disruption. Therefore, ES cells may have a special need for the activity of pol ζ if certain types of DNA damage accumulate within them, and bypass of specific types of DNA lesions by pol ζ is essential for cell viability during embryonic development in mammals.
However, it is also possible that mouse mRev3 has an additional unknown function. In view of the fact that yeast scRev7 shares a region of homology, termed the HORMA (Hop1p, Rev7p and Mad2) domain with Mad2, which associates with unattached kinetochores and functions in the spindle assembly mitotic checkpoint[71]. Mad2, Rev3 and Rev7 proteins might therefore have the potential to interact in vitro. It has been demonstrated that human REV7 may interact with hMAD2[54]. Interestingly, the tumor suppressor p33ING1, which has recently been demonstrated to have no synergistic effect with p53 in camptothecin-induced cell death in melanoma cells[72], strongly associates with mRev3 protein in a two-hybrid assay[30]. It was observed that mouse mRev3-/- embryonic death occurred in a p53-independent pathway indicating mRev3 functions with no direct or indirect interaction with p53[67]. Furthermore, it has been found that Saccharomyces cerevisiae lacking Snm1, scRev3 or Rad51 have a normal S-phase but arrest permanently in G2 after cisplatin treatment[42]. Therefore, pol ζ can play a central role in apoptosis, cell proliferation, and the control of cell cycle by protein-protein interaction, and thus affect the embryonic development.
Edited by Wu XN and Wang XL
| 1. | Li Y, Su JJ, Qin LL, Yang C, Luo D, Ban KC, Kensler T, Roebuck B. Chemopreventive effect of oltipraz on AFB(1)-induced hepatocarcinogenesis in tree shrew model. World J Gastroenterol. 2000;6:647-650. [PubMed] |
| 2. | Bollschweiler E, Hölscher AH. [Carcinoma of the esophagus--actual epidemiology in Germany]. Onkologie. 2001;24:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Bonnin-Scaon S, Lafon P, Chasseigne G, Mullet E, Sorum PC. Learning the relationship between smoking, drinking alcohol and the risk of esophageal cancer. Health Educ Res. 2002;17:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Cai L, Zheng ZL, Zhang ZF. Risk factors for the gastric cardia cancer: a case-control study in Fujian Province. World J Gastroenterol. 2003;9:214-218. [PubMed] |
| 5. | Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinicopathologic features in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:250-253. [PubMed] |
| 6. | Wang AH, Sun CS, Li LS, Huang JY, Chen QS. Relationship of tobacco smoking CYP1A1 GSTM1 gene polymorphism and esophageal cancer in Xi'an. World J Gastroenterol. 2002;8:49-53. [PubMed] |
| 7. | Balaram P, Sridhar H, Rajkumar T, Vaccarella S, Herrero R, Nandakumar A, Ravichandran K, Ramdas K, Sankaranarayanan R, Gajalakshmi V. Oral cancer in southern India: the influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002;98:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Bartal M. Health effects of tobacco use and exposure. Monaldi Arch Chest Dis. 2001;56:545-554. [PubMed] |
| 9. | Boring CC, Squires TS, Tong T, Heath CW. Mortality trends for selected smoking-related cancers and breast cancer--United States, 1950-1990. MMWR Morb Mortal Wkly Rep. 1993;42:857, 863-866. [PubMed] |
| 10. | Bollschweiler E, Hölscher AH. [Carcinoma of the esophagus--actual epidemiology in Germany]. Onkologie. 2001;24:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Badawi AF, Habib SL, Mohammed MA, Abadi AA, Michael MS. Influence of cigarette smoking on prostaglandin synthesis and cyclooxygenase-2 gene expression in human urinary bladder cancer. Cancer Invest. 2002;20:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Bernardini S, Adessi GL, Chezy E, Billerey C, Carbillet JP, Bittard H. Influence of cigarette smoking on P53 gene mutations in bladder carcinomas. Anticancer Res. 2001;21:3001-3004. [PubMed] |
| 13. | Borràs J, Borràs JM, Galceran J, Sánchez V, Moreno V, González JR. Trends in smoking-related cancer incidence in Tarragona, Spain, 1980-96. Cancer Causes Control. 2001;12:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominguez M, Crowder JS, Ross RK, Yu MC. Gender- and smoking-related bladder cancer risk. J Natl Cancer Inst. 2001;93:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Chao A, Thun MJ, Henley SJ, Jacobs EJ, McCullough ML, Calle EE. Cigarette smoking, use of other tobacco products and stomach cancer mortality in US adults: The Cancer Prevention Study II. Int J Cancer. 2002;101:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Casimiro C. [Etiopathogenic factors in colorectal cancer. Nutritional and life-style aspects. 2]. Nutr Hosp. 2002;17:128-138. [PubMed] |
| 17. | Kunz BA, Straffon AF, Vonarx EJ. DNA damage-induced mutation: tolerance via translesion synthesis. Mutat Res. 2000;451:169-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Li Z, Xiao W, McCormick JJ, Maher VM. Identification of a protein essential for a major pathway used by human cells to avoid UV- induced DNA damage. Proc Natl Acad Sci U S A. 2002;99:4459-4464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Yu Y, Chen X. Evidence for nontargeted mutagenesis in a monkey kidney cell line and analysis of its sequence specificity using a shuttle-vector plasmid. Mutat Res. 1994;323:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Quah SK, von Borstel RC, Hastings PJ. The origin of spontaneous mutation in Saccharomyces cerevisiae. Genetics. 1980;96:819-839. [PubMed] |
| 21. | Morrison A, Christensen RB, Alley J, Beck AK, Bernstine EG, Lemontt JF, Lawrence CW. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J Bacteriol. 1989;171:5659-5667. [PubMed] |
| 22. | Nelson JR, Lawrence CW, Hinkle DC. Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science. 1996;272:1646-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 506] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Baynton K, Bresson-Roy A, Fuchs RP. Analysis of damage tolerance pathways in Saccharomyces cerevisiae: a requirement for Rev3 DNA polymerase in translesion synthesis. Mol Cell Biol. 1998;18:960-966. [PubMed] |
| 24. | Lawrence CW, Maher VM. Eukaryotic mutagenesis and translesion replication dependent on DNA polymerase zeta and Rev1 protein. Biochem Soc Trans. 2001;29:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Rechkoblit O, Zhang Y, Guo D, Wang Z, Amin S, Krzeminsky J, Louneva N, Geacintov NE. trans-Lesion synthesis past bulky benzo[a]pyrene diol epoxide N2-dG and N6-dA lesions catalyzed by DNA bypass polymerases. J Biol Chem. 2002;277:30488-30494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Roche H, Gietz RD, Kunz BA. Specificity of the yeast rev3 delta antimutator and REV3 dependency of the mutator resulting from a defect (rad1 delta) in nucleotide excision repair. Genetics. 1994;137:637-646. [PubMed] |
| 27. | Lawrence CW, Hinkle DC. DNA polymerase zeta and the control of DNA damage induced mutagenesis in eukaryotes. Cancer Surv. 1996;28:21-31. [PubMed] |
| 28. | Holbeck SL, Strathern JN. A role for REV3 in mutagenesis during double-strand break repair in Saccharomyces cerevisiae. Genetics. 1997;147:1017-1024. [PubMed] |
| 29. | Xiao W, Fontanie T, Bawa S, Kohalmi L. REV3 is required for spontaneous but not methylation damage-induced mutagenesis of Saccharomyces cerevisiae cells lacking O6-methylguanine DNA methyltransferase. Mutat Res. 1999;431:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Lawrence CW, Maher VM. Mutagenesis in eukaryotes dependent on DNA polymerase zeta and Rev1p. Philos Trans R Soc Lond B Biol Sci. 2001;356:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Gibbs PE, McGregor WG, Maher VM, Nisson P, Lawrence CW. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase zeta. Proc Natl Acad Sci U S A. 1998;95:6876-6880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Diaz M, Velez J, Singh M, Cerny J, Flajnik MF. Mutational pattern of the nurse shark antigen receptor gene (NAR) is similar to that of mammalian Ig genes and to spontaneous mutations in evolution: the translesion synthesis model of somatic hypermutation. Int Immunol. 1999;11:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Diaz M, Verkoczy LK, Flajnik MF, Klinman NR. Decreased frequency of somatic hypermutation and impaired affinity maturation but intact germinal center formation in mice expressing antisense RNA to DNA polymerase zeta. J Immunol. 2001;167:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Zan H, Komori A, Li Z, Cerutti A, Schaffer A, Flajnik MF, Diaz M, Casali P. The translesion DNA polymerase zeta plays a major role in Ig and bcl-6 somatic hypermutation. Immunity. 2001;14:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Zhu F, Jin CX, Song T, Yang J, Guo L, Yu YN. Response of human REV3 gene to gastric cancer inducing carcinogen N-methyl-N'-nitro-N-nitrosoguanidine and its role in mutagenesis. World J Gastroenterol. 2003;9:888-893. [PubMed] |
| 36. | Sherenesheva NI, Mashkovtsev IuV. [Electron microscopy study of experimental stomach cancer]. Eksp Onkol. 1985;7:29-35. [PubMed] |
| 37. | Sasako M. [The effect of Nd: YAG laser irradiation on gastric cancer in rats induced by N-methyl-N'-nitro-N-nitrosoguanidine as a model of endoscopic laser treatment for early gastric cancers]. Nihon Geka Gakkai Zasshi. 1985;86:443-454. [PubMed] |
| 38. | Newberne PM, Charnley G, Adams K, Cantor M, Suphakarn V, Roth D, Schrager TF. Gastric carcinogenesis: a model for the identification of risk factors. Cancer Lett. 1987;38:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Yamashita S, Wakazono K, Sugimura T, Ushijima T. Profiling and selection of genes differentially expressed in the pylorus of rat strains with different proliferative responses and stomach cancer susceptibility. Carcinogenesis. 2002;23:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Amberger H. Different autochthonous models of colorectal cancer in the rat. J Cancer Res Clin Oncol. 1986;111:157-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Narayan S, Jaiswal AS. Activation of adenomatous polyposis coli (APC) gene expression by the DNA-alkylating agent N-methyl-N'-nitro-N-nitrosoguanidine requires p53. J Biol Chem. 1997;272:30619-30622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Grossmann KF, Ward AM, Moses RE. Saccharomyces cerevisiae lacking Snm1, Rev3 or Rad51 have a normal S-phase but arrest permanently in G2 after cisplatin treatment. Mutat Res. 2000;461:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Bemark M, Khamlichi AA, Davies SL, Neuberger MS. Disruption of mouse polymerase zeta (Rev3) leads to embryonic lethality and impairs blastocyst development in vitro. Curr Biol. 2000;10:1213-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Esposito G, Godindagger I, Klein U, Yaspo ML, Cumano A, Rajewsky K. Disruption of the Rev3l-encoded catalytic subunit of polymerase zeta in mice results in early embryonic lethality. Curr Biol. 2000;10:1221-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Wittschieben J, Shivji MK, Lalani E, Jacobs MA, Marini F, Gearhart PJ, Rosewell I, Stamp G, Wood RD. Disruption of the developmentally regulated Rev3l gene causes embryonic lethality. Curr Biol. 2000;10:1217-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Braithwaite DK, Ito J. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res. 1993;21:787-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 466] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Wong SW, Wahl AF, Yuan PM, Arai N, Pearson BE, Arai K, Korn D, Hunkapiller MW, Wang TS. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988;7:37-47. [PubMed] |
| 48. | Pavlov YI, Shcherbakova PV, Kunkel TA. In vivo consequences of putative active site mutations in yeast DNA polymerases alpha, epsilon, delta, and zeta. Genetics. 2001;159:47-64. [PubMed] |
| 49. | Kajiwara K, Nagawawa H, Shimizu-Nishikawa S, Ookuri T, Kimura M, Sugaya E. Molecular characterization of seizure-related genes isolated by differential screening. Biochem Biophys Res Commun. 1996;219:795-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Morelli C, Mungall AJ, Negrini M, Barbanti-Brodano G, Croce CM. Alternative splicing, genomic structure, and fine chromosome localization of REV3L. Cytogenet Cell Genet. 1998;83:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Lin W, Wu X, Wang Z. A full-length cDNA of hREV3 is predicted to encode DNA polymerase zeta for damage-induced mutagenesis in humans. Mutat Res. 1999;433:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Van Sloun PP, Romeijn RJ, Eeken JC. Molecular cloning, expression and chromosomal localisation of the mouse Rev3l gene, encoding the catalytic subunit of polymerase zeta. Mutat Res. 1999;433:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Eeken JC, Romeijn RJ, de Jong AW, Pastink A, Lohman PH. Isolation and genetic characterisation of the Drosophila homologue of (SCE)REV3, encoding the catalytic subunit of DNA polymerase zeta. Mutat Res. 2001;485:237-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Murakumo Y, Roth T, Ishii H, Rasio D, Numata S, Croce CM, Fishel R. A human REV7 homolog that interacts with the polymerase zeta catalytic subunit hREV3 and the spindle assembly checkpoint protein hMAD2. J Biol Chem. 2000;275:4391-4397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 55. | Murakumo Y, Ogura Y, Ishii H, Numata S, Ichihara M, Croce CM, Fishel R, Takahashi M. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J Biol Chem. 2001;276:35644-35651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 180] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases iota and zeta act sequentially to bypass DNA lesions. Nature. 2000;406:1015-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 526] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 57. | Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 470] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 58. | Woodgate R. Evolution of the two-step model for UV-mutagenesis. Mutat Res. 2001;485:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Maenhaut-Michel G. Mechanism of SOS-induced targeted and untargeted mutagenesis in E. coli. Biochimie. 1985;67:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Pham P, Bertram JG, O'Donnell M, Woodgate R, Goodman MF. A model for SOS-lesion-targeted mutations in Escherichia coli. Nature. 2001;409:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Otterlei M, Kavli B, Standal R, Skjelbred C, Bharati S, Krokan HE. Repair of chromosomal abasic sites in vivo involves at least three different repair pathways. EMBO J. 2000;19:5542-5551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Kim SR, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, Nohmi T, Ohmori H. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc Natl Acad Sci U S A. 1997;94:13792-13797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 269] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 63. | Tang M, Bruck I, Eritja R, Turner J, Frank EG, Woodgate R, O'Donnell M, Goodman MF. Biochemical basis of SOS-induced mutagenesis in Escherichia coli: reconstitution of in vitro lesion bypass dependent on the UmuD'2C mutagenic complex and RecA protein. Proc Natl Acad Sci U S A. 1998;95:9755-9760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Tang M, Pham P, Shen X, Taylor JS, O'Donnell M, Woodgate R, Goodman MF. Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature. 2000;404:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 347] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 65. | Lawrence CW, Christensen RB. The mechanism of untargeted mutagenesis in UV-irradiated yeast. Mol Gen Genet. 1982;186:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Boesen JJ, Stuivenberg S, Thyssens CH, Panneman H, Darroudi F, Lohman PH, Simons JW. Stress response induced by DNA damage leads to specific, delayed and untargeted mutations. Mol Gen Genet. 1992;234:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | O-Wang J, Kajiwara K, Kawamura K, Kimura M, Miyagishima H, Koseki H, Tagawa M. An essential role for REV3 in mammalian cell survival: absence of REV3 induces p53-independent embryonic death. Biochem Biophys Res Commun. 2002;293:1132-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1053] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 69. | Van Sloun PP, Jansen JG, Weeda G, Mullenders LH, van Zeeland AA, Lohman PH, Vrieling H. The role of nucleotide excision repair in protecting embryonic stem cells from genotoxic effects of UV-induced DNA damage. Nucleic Acids Res. 1999;27:3276-3282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Copp AJ. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 279] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 71. | Aravind L, Koonin EV. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem Sci. 1998;23:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Cheung KJ, Li G. The tumour suppressor p33ING1 does not enhance camptothecin-induced cell death in melanoma cells. Int J Oncol. 2002;20:1319-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |