Published online May 15, 2003. doi: 10.3748/wjg.v9.i5.974
Revised: November 27, 2002
Accepted: December 22, 2002
Published online: May 15, 2003
AIM: To identify the role of survivin in colorectal carcinogenesis and the relationship between Survivin and histological differentiation grade of colorectal carcinoma.
METHODS: Immunohistochemical staining of survivin by using the monoclonal antibody was performed by the standard streptavidin-peroxidase (SP) technique for the 188 paraffin sections which included 30 normal colorectal mucosas, 41 adenomas with low grade dysplasia, 30 adenomas with high grade dysplasia, and 87 colorectal carcinomas which were classified as high, middle and low differentiated subgroups which included 33, 28, 26 cases respectively.
RESULTS: Expression of survivin was observed in the cytoplasm of adenoma with dysplasia and colorectal carcinoma cells. No immunoreactivity of survivin was seen in normal mucosas. The positive rate of survivin increased in the transition from normal mucosas to adenomas with low grade dysplasia to high grade dysplasia/carcinomas (0.0%, 31.7%, 56.7% and 63.2% respectively). But the difference between high grade dysplasia and carcinomas had no statistical significance. Positive rate was not related to histological differentiation grade of colorectal carcinoma. Moreover, there was no correlation between histological differentiation grade of colorectal carcinoma and immunoreactive intensity of survivin.
CONCLUSION: The expression of survivin is the essential event in the early stage of colorectal carcinogenesis and plays an important role in the transition sequence and it is not related to histological differentiation grade of colorectal carcinoma. It thus may provide a new diagnostic and therapeutic target in colorectal cancer.
- Citation: Lin LJ, Zheng CQ, Jin Y, Ma Y, Jiang WG, Ma T. Expression of survivin protein in human colorectal carcinogenesis. World J Gastroenterol 2003; 9(5): 974-977
- URL: https://www.wjgnet.com/1007-9327/full/v9/i5/974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i5.974
Disturbance of apoptosis is thought to be very important in neoplastic transformation and progression. Several tumor suppressor genes and oncogenes, such as p53 and the bcl-2 family, are involved in regulation of cell apoptosis, which were thoroughly studied[1-12]. Moreover, a gene family of inhibitor of apoptosis (IAP) has been identified recently. Survivin, a novel member of IAP family, directly inhibits caspase-3 and -7 activity[13] or conjugates caspase-9[14], and regulates the cell cycle in the G2/M phase by interact with spindle microtubules[15]. Survivin shows markedly different tissue expression compared with other IAPs. It is present during embryonic and fetal development, but is downregulated in normal adult tissues. However, it becomes re-expressed in a variety of cancers[16-20]. The unique feature makes it attractive as a target for cancer therapy[21]. In this study, we sought to investigate the expression of survivin in normal colorectal mucosas, adenomas with low grade dysplasia, adenomas with high grade dysplasia, and colorectal carcinomas by immunohistochemical staining method in order to identify the role of survivin in colorectal carcinogenesis and the relationship between survivin and histological differentiation grade of colorectal carcinoma.
Tissue specimens used for this study were obtained from 188 patients which were resected surgically or endoscopically at the 2nd Hospital Affiliated to China Medical University from 1998 to 2002. There were 105 males and 83 females, and the mean age of the patients was 56.2 years. Materials were composed of 30 cases of normal colorectal mucosas, 41 cases of adenomas with low grade dysplasia, 30 cases of adenomas with high grade dysplasia, and 87 cases of colorectal carcinomas, their mean ages were 52.3, 55.4, 57.9 and 57.4 respectively. According to histological differentiation grade, 87 cases of colorectal carcinoma were classified to high, middle and low differentiated subgroups which included 33, 28, 26 cases and the mean ages were 62.0, 57.9, and 51.1 respectively. The patients had received neither chemotherapy nor radiation therapy before tumor resection.
Routinely processed formalin fixed, paraffin embedded, serial sections of 5 µm were prepared from the cut surface of blocks at the maximum cross-section. For morphologic analysis, tissue sections were routinely stained with hematoxylin and eosin. At least 2 experienced pathologists studied the sections. The immunohisto-chemical staining for survivin antigen was carried out by the standard streptavidin/peroxidase (SP) technique. Briefly, before labeling with primary antibody, paraffin sections were dewaxed, and then incubated with 30 ml/L hydrogen peroxide for 10 minutes, and antigen retrieval was performed by boiling in EDTA (0.01M, pH7.4). The sections were cooled and washed in PBS. Nonspecific reactions were blocked by incubating the sections in a solution containing normal serum. The sections were incubated with a primary antibody overnight at 4 °C. The anti-survivin antibody was sc-8806 antibody (Sant Cruz Biotechnology, Inc) at a 1:50 dilution. Rinsed with PBS, then the sections were incubated for 30 minutes at 37 °C with biotinylated secondary antibody and streptavidin conjugated to horseradish peroxidase, respectively. After three rinses with PBS, the sections were incubated with diaminobenzidine substrate, then rinsed with distilled water and counterstained with hematoxylin.
The mean percentage of positive cells for the expression of survivin was determined in at least 5 areas at 400-fold magnification, and cases with less than 10% positively stained cells were defined as negative. Cases with 10 to 29% positively stained cells were defined as "+", 30 to 59% as "++", and 60% or more than 60% as "+++". These scorings were performed in a blinded fashion.
The difference and correlation were analyzed by χ2 test. A value of P < 0.05 was considered statistically significant.
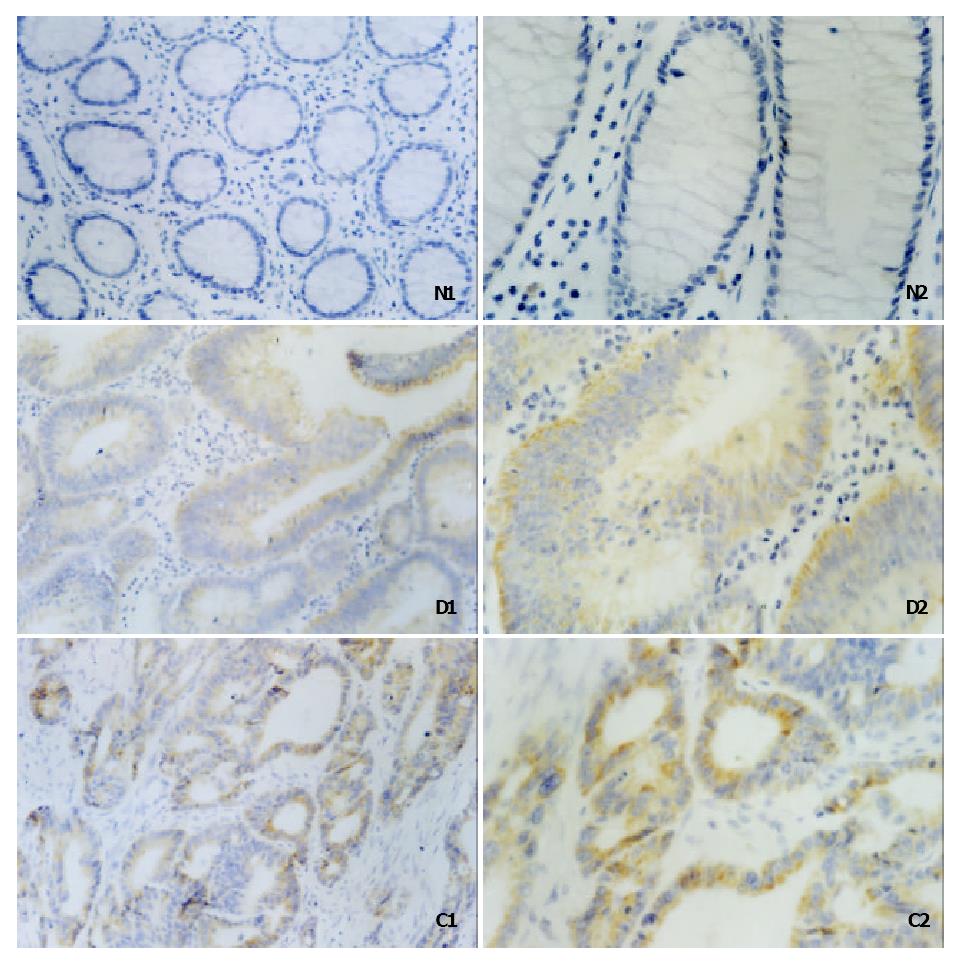
By immunohistochemical staining, we examined the expression of survivin in adenoma-carcinoma sequence. Representative results were shown in Figure 1. The expression of survivin was observed in the cytoplasm of the benign and malignant tumor cells, whereas not in normal tissues. As shown in Table 1, the positive rate for survivin expression increased gradually from normal colorectal mucosas to adenomas with low grade dysplasia, adenomas with high grade dysplasia, and carcinomas. The expression rates were 0.0%, 31.7%, 56.7%, and 63.2% respectively. Analyzed by χ2 test, there were significant differences in the expressions of survivin between the normal mucosas group and any one of the other groups, between adenomas with low grade dysplasia and carcinomas, and between adenomas with low grade dysplasia and adenomas with high grade dysplasia (P < 0.05), while there were no significant differences between adenomas with high grade dysplasia and carcinomas (χ2 = 0.40, 0.5 < P < 0.75).
| Lesion | n | Expression intensity | Expression rate% | |||
| - | + | ++ | +++ | |||
| Normal mucosas | 30 | 30 | 0 | 0 | 0 | 0.0 |
| Adenomas with low grade dysplasia | 41 | 28 | 7 | 5 | 0 | 31.7 |
| Adenomas with high grade dysplasia | 30 | 13 | 3 | 7 | 7 | 56.7 |
| Carcinomas | 87 | 32 | 8 | 20 | 27 | 63.2 |
To study the relationship between survivin and histological differentiation grade of colorectal carcinoma, 87 cases of colorectal carcinoma were classified to high, middle and low differentiated subgroups. The results were shown in Table 2. Analyzed by χ2 test, there were no significant differences in the expressions of survivin among the subgroups (P > 0.90). Mo reo ver, there was no relation ship between the differentiation grade of colorectal carcinoma and the expression intensity (P > 0.75).
| Lesion | n | Expression intensity | Expression rate% | |||
| - | + | ++ | +++ | |||
| High differentiation | 33 | 12 | 3 | 10 | 8 | 63.6 |
| Middle differentiation | 28 | 11 | 2 | 4 | 11 | 60.7 |
| Low differentiation | 26 | 9 | 3 | 6 | 8 | 65.4 |
| Total | 87 | 32 | 8 | 20 | 27 | 63.2 |
The development of colorectal carcinoma proceeds through a series of genetic changes involving the activation of oncogenes and loss of tumor suppressor genes. During this process, a disturbance in the balance between cell proliferation and apoptosis may underlie neoplastic development. Previous investigations have well studied the role of p53 and bcl-2 family[1-12]. The IAPs is a widely expressed gene family of apoptosis inhibitors. Survivin, a novel and structurally unique member of the IAP gene family, is the strongest apoptosis inhibitor. A characteristic finding of survivin is that it is expressed during embryonic and fetal development but not in normal adult differentiated tissues, and prominently reexpressed in the most common human carcinomas. The mechanism is unclear. In this study, we aimed to identify the role of survivin in colorectal carcinogenesis and the relationship between survivin and histological differentiation grade of colorectal carcinoma.
In our study, we demonstrated that survivin was not expressed in normal colorectal mucosas, which coincided with previous reports. The expression of survivin was localized in the cytoplasm of the adenoma and carcinoma cells, and the positive rate for survivin expression increased gradually from normal colorectal mucosas to adenomas with low grade dysplasia, adenomas with high grade dysplasia, and to carcinomas. The expression rates were 0.0%, 31.7%, 56.7% and 63.2% respectively. Analyzed by χ2 test, there were significant differences in the expressions of survivin between the normal mucosa group and any one of the other groups (P < 0.05), between adenomas with low grade dysplasia and carcinomas (P < 0.05), and between adenomas with low grade dysplasia and adenomas with high grade dysplasia, while there were no significant differences between adenomas with high grade dysplasia and carcinomas. We analyze the adenomas with high grade dysplasia and the carcinoma groups. The positive rates of them were 56.7% and 63.2% respectively, and the total number of cases were 30 and 87 respectively, then, the value of χ2 was 0.40 and P was more than 0.5 but less than 0.75. Thus, we consider that there is no difference between them. This result has not been analyzed by other studies, but it coincides with the clinical practice that the treatment of adenomas with high grade dysplasia is similar to that of the carcinomas.
To study the relationship between survivin and histological differentiation grade of colorectal carcinoma, 87 cases of colorectal carcinoma were classified to high, middle and low differentiated subgroups. The number of cases were 33, 28, and 26 respectively, and the positive rates were 63.6%, 60.7% and 65.4% respectively. Analyzed by χ2 test, there was no significant difference in the expressions of survivin among the subgroups (P > 0.90). Moreover, there was no relationship between the differentiation grade of colorectal carcinoma and the expression intensity (P > 0.75). These results conflict with those of Wang Mei et al[22], who thought the expression of survivin was related to the tumor histological grade in cervical carcinoma. These may be due to the different tissue origin. There was no significant difference in the expressions of survivin among the different grade of colorectal carcinoma, which makes it possible to use survivin as a tumor-specific target for therapy or diagnosis. That is, no matter what historical grade the colorectal carcinoma is, the cancer can be diagnosed by detecting survivin and be treated by targeting survivin. Meanwhile, the normal cells will not be killed because survivin is not expressed in normal cells. Survivin is an attractive candidate for cancer therapy. Therefore, our study gives some directions to diagnose and treat colorectal cancer. Whether the expression of survivin can predict prognosis in cancer or not is still under discussion[23-36].
Survivin, a novel mammalian IAP molecule, has interested scholars for its unique developmentally regulated expression and mechanism. There are still many problems to be solved, such as the molecular mechanism for its selective expression, the details about its anti-apoptosis, and so on. The targeting therapy is just beginning. Therefore, it is worthy to be further studied to settle a firm basis of tumor diagnosis and therapy.
Edited by Xu JY
| 1. | Lan J, Xiong YY, Lin YX, Wang BC, Gong LL, Xu HS, Guo GS. Helicobacter pylori infection generated gastric cancer through p53-Rb tumor-suppressor system mutation and telomerase reactivation. World J Gastroenterol. 2003;9:54-58. [PubMed] |
| 2. | Jain D, Srinivasan R, Patel FD, Kumari Gupta S. Evaluation of p53 and Bcl-2 expression as prognostic markers in invasive cervical carcinoma stage IIb/III patients treated by radiotherapy. Gynecol Oncol. 2003;88:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Kim KY, Seol JY, Jeon GA, Nam MJ. The combined treatment of aspirin and radiation induces apoptosis by the regulation of bcl-2 and caspase-3 in human cervical cancer cell. Cancer Lett. 2003;189:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Pepper C, Thomas A, Hoy T, Bentley P. Antisense oligonucleotides complementary to Bax transcripts reduce the susceptibility of B-cell chronic lymphocytic leukaemia cells to apoptosis in a bcl-2 independent manner. Leuk Lymphoma. 2002;43:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Iyer R, Ding L, Batchu RB, Naugler S, Shammas MA, Munshi NC. Antisense p53 transduction leads to overexpression of bcl-2 and dexamethasone resistance in multiple myeloma. Leuk Res. 2003;27:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Selim AG, El-Ayat G, Wells CA. Expression of c-erbB2, p53, Bcl-2, Bax, c-myc and Ki-67 in apocrine metaplasia and apocrine change within sclerosing adenosis of the breast. Virchows Arch. 2002;441:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Choi YH, Kim MJ, Lee SY, Lee YN, Chi GY, Eom HS, Kim ND, Choi BT. Phosphorylation of p53, induction of Bax and activation of caspases during beta-lapachone-mediated apoptosis in human prostate epithelial cells. Int J Oncol. 2002;21:1293-1299. [PubMed] |
| 8. | Kasimir-Bauer S, Beelen D, Flasshove M, Noppeney R, Seeber S, Scheulen ME. Impact of the expression of P glycoprotein, the multidrug resistance-related protein, bcl-2, mutant p53, and heat shock protein 27 on response to induction therapy and long-term survival in patients with de novo acute myeloid leukemia. Exp Hematol. 2002;30:1302-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Rincheval V, Renaud F, Lemaire C, Godefroy N, Trotot P, Boulo V, Mignotte B, Vayssière JL. Bcl-2 can promote p53-dependent senescence versus apoptosis without affecting the G1/S transition. Biochem Biophys Res Commun. 2002;298:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Sakuragi N, Salah-eldin AE, Watari H, Itoh T, Inoue S, Moriuchi T, Fujimoto S. Bax, Bcl-2, and p53 expression in endometrial cancer. Gynecol Oncol. 2002;86:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Nagler RM, Kerner H, Laufer D, Ben-Eliezer S, Minkov I, Ben-Itzhak O. Squamous cell carcinoma of the tongue: the prevalence and prognostic roles of p53, Bcl-2, c-erbB-2 and apoptotic rate as related to clinical and pathological characteristics in a retrospective study. Cancer Lett. 2002;186:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-kB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431-435. [PubMed] |
| 13. | Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 527] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 14. | O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000;97:13103-13107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 497] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 15. | Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462-2467. [PubMed] |
| 16. | Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Mori A, Wada H, Nishimura Y, Okamoto T, Takemoto Y, Kakishita E. Expression of the antiapoptosis gene survivin in human leukemia. Int J Hematol. 2002;75:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Das A, Tan WL, Teo J, Smith DR. Expression of survivin in primary glioblastomas. J Cancer Res Clin Oncol. 2002;128:302-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Lehner R, Lucia MS, Jarboe EA, Orlicky D, Shroyer AL, McGregor JA, Shroyer KR. Immunohistochemical localization of the IAP protein survivin in bladder mucosa and transitional cell carcinoma. Appl Immunohistochem Mol Morphol. 2002;10:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Sasaki T, Lopes MB, Hankins GR, Helm GA. Expression of survivin, an inhibitor of apoptosis protein, in tumors of the nervous system. Acta Neuropathol. 2002;104:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Yamamoto T, Tanigawa N. The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001;34:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Wang M, Wang B, Wang X. [A novel antiapoptosis gene, survivin, bcl-2, p53 expression in cervical carcinomas]. Zhonghua Fuchanke Zazhi. 2001;36:546-548. [PubMed] |
| 23. | Nakanishi K, Tominaga S, Hiroi S, Kawai T, Aida S, Kasamatsu H, Aurues T, Hayashi T, Ikeda T. Expression of survivin does not predict survival in patients with transitional cell carcinoma of the upper urinary tract. Virchows Arch. 2002;441:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Rödel F, Hoffmann J, Grabenbauer GG, Papadopoulos T, Weiss C, Günther K, Schick C, Sauer R, Rödel C. High survivin expression is associated with reduced apoptosis in rectal cancer and may predict disease-free survival after preoperative radiochemotherapy and surgical resection. Strahlenther Onkol. 2002;178:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Dong Y, Sui L, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression in laryngeal squamous cell carcinomas and its prognostic implications. Anticancer Res. 2002;22:2377-2383. [PubMed] |
| 26. | Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol. 2002;21:315-320. [PubMed] |
| 27. | Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett. 2002;184:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Ikehara M, Oshita F, Kameda Y, Ito H, Ohgane N, Suzuki R, Saito H, Yamada K, Noda K, Mitsuda A. Expression of survivin correlated with vessel invasion is a marker of poor prognosis in small adenocarcinoma of the lung. Oncol Rep. 2002;9:835-838. [PubMed] |
| 29. | Würl P, Kappler M, Meye A, Bartel F, Köhler T, Lautenschläger C, Bache M, Schmidt H, Taubert H. Co-expression of survivin and TERT and risk of tumour-related death in patients with soft-tissue sarcoma. Lancet. 2002;359:943-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Sandler A, Scott D, Azuhata T, Takamizawa S, O'Dorisio S. The survivin: Fas ratio is predictive of recurrent disease in neuroblastoma. J Pediatr Surg. 2002;37:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ikeguchi M, Ueda T, Sakatani T, Hirooka Y, Kaibara N. Expression of survivin messenger RNA correlates with poor prognosis in patients with hepatocellular carcinoma. Diagn Mol Pathol. 2002;11:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, Loeffler JS. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Sarela AI, Scott N, Ramsdale J, Markham AF, Guillou PJ. Immunohistochemical detection of the anti-apoptosis protein, survivin, predicts survival after curative resection of stage II colorectal carcinomas. Ann Surg Oncol. 2001;8:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Tajiri T, Tanaka S, Shono K, Kinoshita Y, Fujii Y, Suita S, Ihara K, Hara T. Quick quantitative analysis of gene dosages associated with prognosis in neuroblastoma. Cancer Lett. 2001;166:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Kato J, Kuwabara Y, Mitani M, Shinoda N, Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer. 2001;95:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 36. | Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, Takano Y. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 2001;163:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |