INTRODUCTION
Alginate microcapsules have been used extensively for different applications, particularly for the encapsulation of pancreatic islet cells and insulin delivery[1]. This method has also been used for the encapsulation of cells that release growth hormone, β-endorphin, endostatin and other agents for gene therapy[2-5]. The alginate membranes allow the free exchange of nutrients and oxygen between the implanted cells, and could prevent the escape and elimination of encapsulated cells. More important, this approach provides a prolonged sustained delivery of recombinant protein produced by the cells, thus maintaining high levels of the agent.
In recent years, interleukine-12 (IL-12) has received considerable interest in cancer biologic therapy. In vivo IL-12 was found to have a potent antitumor efficacy in a variety of murine tumor models[6,7]. Local or systemic treatment with recombinant IL-12 protein (rIL-12) was shown to inhibit the growth of established subcutaneous tumor and tumor metastasis[8-10]. However, systemic administration of rIL-12 caused severe dose-dependent toxicity and led to an interruption of the first human trial[11]. In contrast, the local transfer of cytokine genes as a means for gene therapy could circumvent such systemic toxicity and provide effective and persistent local cytokine levels for immune cells activation[12-15]. Some studies using an ex vivo IL-12 gene therapy yielded encouraging results, showing that murine fibroblasts or tumor cells transduced in vitro with IL-12 cDNA, using a retroviral vector, were able to induce antitumor immune responses in the absence of apparent toxicities[16]. This strategy, however, has many obstacles precluding successful clinical application: e.g. autologous somatic cells or tumor cells are difficult to culture and transfect, and selection for transfected cells requires prolonged culture and the attendant costs of these process are expensive. To avoid these potential disadvantages, an alternative approach to obtain prolonged local cytokine secretion is adopted to use microencapsulated engineered cells to secrete IL-12.
In the present study, NIH3T3 cells engineered to continuously secrete high levels of mIL-12 were encapsulated with alginate. The ability of this system to secrete biologically active mIL-12 capable of inhibiting the tumor growth of a murine colon carcinoma xenograft in the mouse was investigated.
MATERIALS AND METHODS
Mice and cell lines
Male BALB/C mice aged between 6 and 8 weeks were purchased from Joint Ventures Sipper BK Experimental Animal Company (Shanghai, China) and housed in a specific pathogen-free condition for all experiments. Mouse fibroblasts (NIH3T3) and the murine colon adenocarcinoma cell line (CT26) were donated by the Institute of Immunology, Zhejiang University (Hangzhou, China). Cells were cultured in RPMI-1640 medium (GIBCO-BRL, Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClon, Logan, UT, USA), 2 mM glutamine, penicillin 100 U/ml, and streptomycin 100 μg/ml.
Expression plasmids and transfection of NIH3T3 cells
Murine p35 and p40 subunits of mIL-12 were subcloned into pcDNA3.1 plasmids containing a cytomegalovirus (CMV) immediate-early enhancer promoter and a G418 selected gene. NIH3T3 cells were stably transfected with these expression plasmids using LF2000TM (Ivitrogen, Life Technologies, USA). To obtain stably transfected clones (NIH3T3-mIL-12), transfected cells were grown in G418 containing medium (400 g/L, Ivitrogen, Life Technologies, USA) for 14 days, and resistant clones were propagated separately. With subsequent determination of mIL-12 expression by ELISA kit (R&D systems, Inc., USA).
Microencapsulation of NIH3T3-mIL-12
NIH3T3-mIL-12 cells were encapsulated within microspheres composed of Ba2+-alginate. Briefly, cells were resuspended in sodium alginate-saline (1.5% wt/vol, purified by Syringe Driven Filter Unit) (Sigma, St Louis, MO, USA) to a final ratio of 0.5 × 109 cells/L of alginate. The suspension was sprayed through an air jet-head droplet-forming apparatus, into a solution of 4.9% Barium chloride (pH7.4, Sigma), where they were allowed to gel for 10 min, washed three times with PBS, then cultured in the conditioned medium described above. The number of cells encapsulated and the viability of the cells in the microcapsules were evaluated weekly using a modified MTT assay.
In vitro release of mIL-12 from encapsulated NIH3T3-mIL-12 cells
Microencapsulated NIH3T3-mIL-12 cells were suspended in the conditioned medium described above at a density of 2 × 105 cells/well. The medium was collected every 2 hrs. and assayed for mIL-12 using ELISA assay (Endogen, Woburn, MA, USA). Medium from NIH3T3-mIL-12 monolayer cells was used as a positive control.
Murine studies
The BALB/C mice were inoculated subcutaneously in the right-behind armpit with CT26 cells (2 × 105 tumor cells/injection). Mice were randomly divided into four groups of twenty each. Group 1 received a single subcutaneous injection of microcapsules containing NIH3T3-mIL-12 cells within 0.5 cm apart from the area where CT26 cells were inoculated (1 × 105 encapsulated cells/animal); Group 2 RPMI-1640 (control), Group 3 microcapsules containing NIH3T3 (1 × 105 cells), and Group 4 RPMI injected at the same region of mice. The length and width of the tumor mass were measured with calibers every other day after tumor inoculation. Tumor size was expressed as 1/2 (length + width). Twenty-one days after tumor inoculation, ten mice taken randomly from each group were sacrificed, and spleen was resected, and blood was collected from the mice eyeballs. The rest ten mice in each group were observed for their survival period until 60 days after injection.
Cytotoxic assay of CTL and NK cells
Spleen cells derived from each group of experimental mice were unstimulated or were stimulated with irradiated CT26 cells (1 × 105) for 7 days in vitro and processed for the NK or cytotoxic T lymphocyte (CTL) assay, respectively. The NK activity and CTL activity were determined by lactate dehydrogenase (LDH) release assay with Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA). The target cells (YAC-1 cells for the NK assay, and CT26 cells for the CTL assay) were washed three times with RPMI 1640 medium containing 50 ml·L-1 FCS to remove adherent LDH derived from lysed cells. The cell suspension was diluted with RPMI 1640 medium containing 50 ml·L-1 FCS to give a final concentration of 1 × 108 cells/L; and 100 µl target cell suspension and 100 µl different ratios of effector cells were pipetted together into the wells of a round-bottomed microtiter plate. Suspensions containing exclusively effector cells, target cells, or culture medium, respectively, served as controls to estimate the LDH background. The plates were incubated for 4 hrs in a humidified 50 ml·L-1 CO2 atmosphere at 37 °C. After incubation, they were centrifuged for 10 min. Then 100 µl of the supernatant from each well was transferred to the corresponding well of enzymatic assay plate. Fifty µl reconstituted substrate mix (containing lactate and NAD+) was added to each well. The plate was covered and incubated at room temperature (protected from light). Thirty minutes later, 50 µl stop solution was added to each well. The reaction was measured in an ELISA reader at a wavelength of 490 nm. Calculations were carried out according to the following formula: percent specific lysis = 100 × (mean experimental cpm - mean spontaneous cpm)/(mean maximum cpm - mean spontaneous cpm).
Determination of serum cytokine production
Blood samples were collected from the mice 21 days after tumor inoculation. Stored serum were separated from the whole blood and frozen at -70 °C until analyzed for cytokine production. mIL-2, mIL-12, mIL-4, mIL-10 and mIFN-γ were measured using a standard sandwich ELISA technique with corresponding kits purchased from Endogen (Woburn, MA, USA).
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was performed using the t test and log-rank test (for survival analysis). The difference was considered statistically significant when the P value was less than 0.05. The SPSS software package version 10.0 was used for statistical calculation.
RESULTS
In vitro expression and release of mIL-12 from encapsulated cells
NIH3T3 cells were transfected with a mIL-12 expression vector and clonal populations of stably transfected NIH3T3 cells were obtained (NIH3T3-mIL-12). The microcapsules have an average diameter of 0.45 mm ± 0.05 mm (Figure 1). Both encapsulated and nonencapsulated NIH3T3-mIL-12 cells were cultured in vitro, and the conditioned medium was collected every week for six weeks. The encapsulated cells were viable in culture as determined by MTT assay. ELISA method was used to determine mIL-12 in the medium collected at all time points. The average concentration of mIL-12 secreted by 2 × 105 cultured encapsulated NIH3T3-mIL-12 cells or nonencapsulated NIH3T3-mIL-12 cells were 5.12 µg·L-1 and 5.45 µg·L-1 for every 24hrs, and the optimal expression up to 46.8 and 48.2 ng of mIL-12 per 24 hrs per 105 cells, respectively. These results indicate that mIL-12 protein could release freely from the microencapsulated NIH3T3-mIL-12 cells.
Figure 1 NIH3T3-mIL-12 cells-loaded microcapsules (average capsule diameter 450 µm).
Increased NK activity after delivery of microencapsulated NIH3T3-mIL-12 cells
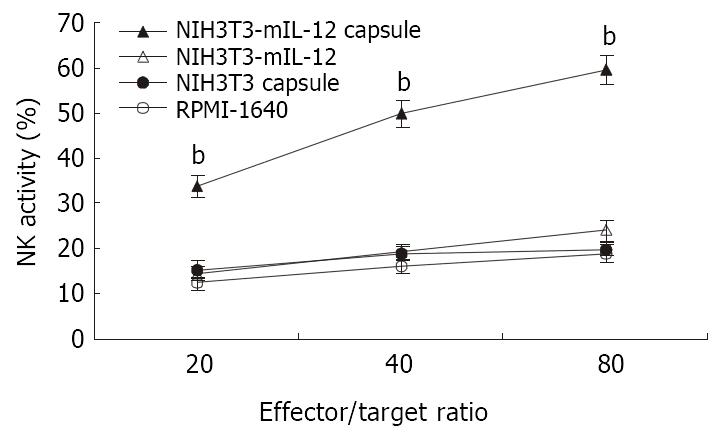
Twenty-one days after treatment of tumor-bearing mice with various injections, the splenocytes were used in cytolytic assay against YAC-1 cells at effector:target (E:T) ratios at 20:1, 40:1, 80:1. As shown in Figure 2, NK activity in mice treated with NIH3T3-mIL-12 cells capsule increased significantly when compared with the mice treated with NIH3T3-mIL-12 cells, NIH3T3 cells capsule or RPMI-1640 (P < 0.01). These data suggested that nonspecific immunity was enhanced significantly by the local delivery of IL-12 with NIH3T3-mIL-12 cells capsule.
Figure 2 NK activity induced by various treatment.
-x±s, n = 10, bP < 0.01 for the group treated with NIH3T3-mIL-12 cells capsule versus other three counterpart groups, respectively.
Potent CTL activity induced by delivery of microencapsulated NIH3T3-mIL-12 cells
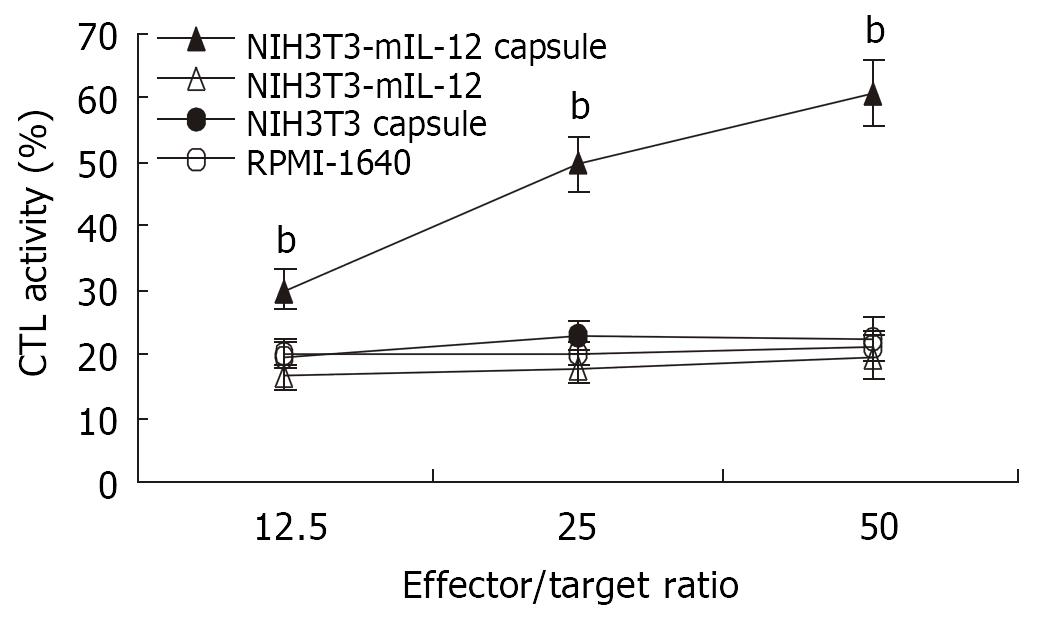
The splenocytes collected from various groups were restimulated in vitro with inactivated CT26 tumor cells for CTL induction. As shown in Figure 3, the mice treated with NIH3T3-mIL-12 cells capsule exhibited a CT26 colon carcinoma-specific CTL response that was higher than that of mice treated with NIH3T3-mIL-12 cells, NIH3T3 cells capsule or RPMI-1640 (P < 0.01). It suggested that CTL activity against tumor was induced significantly by the local delivery of IL-12 with NIH3T3-mIL-12 cells capsule.
Figure 3 CTL activity against CT26 induced by various treatment.
-x±s, n = 10, bP < 0.01 for the group treated with NIH3T3-mIL-12 cells capsule versus other three counterpart groups, respectively.
Serum cytokine production
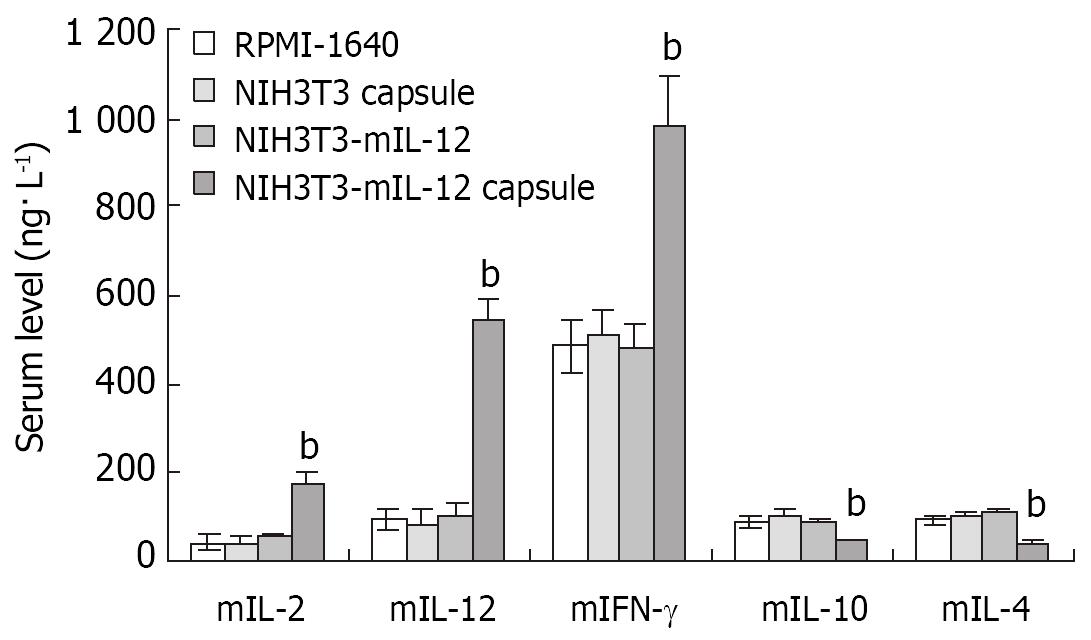
Twenty-one days after the microencapsulated NIH3T3-mIL-12 cells were injected into the tumor-bearing mice, blood was collected for analysis of serum mIL-12, mIL-2, mIFN-γ and mIL-4, mIL-10. The serum average concentrations of mIL-12, mIL-2 an d mIFN-γ in the group treated with microencapsulated NIH3T3-mIL-12 cells were 542 ± 48, 176 ± 25 and 982 ± 112 ng·L-1, respectively, which were significantly higher as compared with the counterpart control groups (P < 0.01), but the serum concentrations of mIL-4 and mIL-10 were lowered significantly compared to the controls (P < 0.01). The results of the studies are shown in Figure 4.
Figure 4 Cytokines levels in serum after various treatment in the tumor-bearing model.
-x±s, n = 10, bP < 0.01 for the group treated with NIH3T3-mIL-12 cells capsule versus other three counterpart groups, respectively.
Effects of encapsulated NIH3T3-mIL-12 cells on subcutaneous tumor xenografts
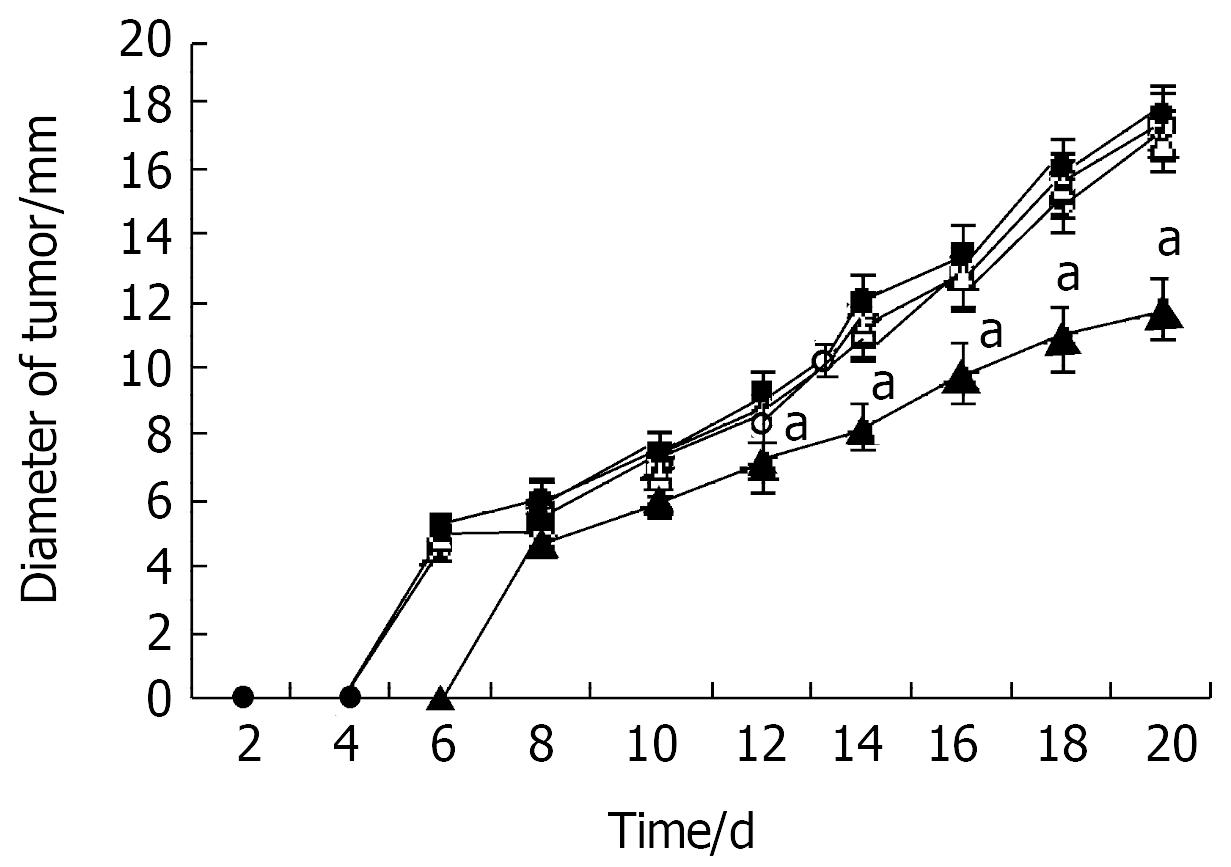
The subcutaneous tumor size was calculated as follows: tumor size = (maximum diameter + vertical diameter)/2. The growth of tumor xenografts was significantly inhibited by a single dose of microcapsules containing NIH3T3-mIL-12 cells when compared with both control groups (P < 0.05) (Figure 5).
Figure 5 Inhibition of tumor growth by microencapsulated NIH3T3-mIL-12.
Two days after tumor inoculation, mice were injected sc with NIH3T3-mIL-12 cells capsule (▲), NIH3T3-mIL-12 cells (△), NIH3T3 cells capsule (●) or RPMI-1640 (○). -x±s, n = 20, aP < 0.05 for the group treated with NIH3T3-mIL-12 cells capsule versus other three counterpart groups respectively.
Survival time after delivery of microencapsulated NIH3T3-mIL-12 cells in the tumor-bearing model
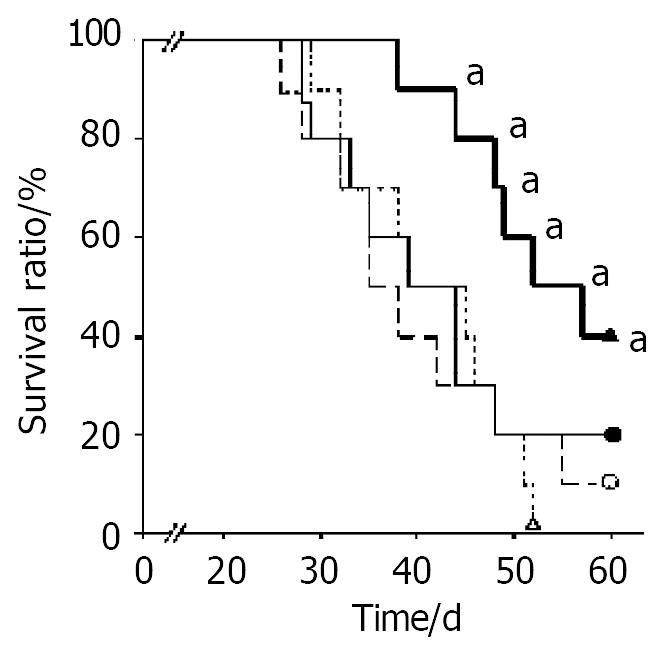
When treated mice were observed up to 60 days after implantation of CT26 tumor, the mice survival time of the group treated with microencapsulated NIH3T3-mIL-12 cells was longer than other counterpart control groups (P < 0.05) (Figure 6).
Figure 6 Survival time after various treatment in the tumor-bearing model.
Two days after tumor inoculation, mice were injected sc with RPMI-1640 (○), NIH3T3 cells capsule (●), NIH3T3-mIL-12 cells (△) and NIH3T3-mIL-12 cells capsule (▲), respectively. Ten tumor-bearing mice (n = 10) in each group were observed for their survival time. All surviving mice were monitored for at least 60 d. aP < 0.05, compared with other three counterpart groups, respectively.
DISCUSSION
Encapsulation of living cells in a protective, biocompatible, and semipermeable polymeric membrane has been proven to be an effective method for immunoprotection of desired cells, regardless of the type recipient involved (allograft, xenograft)[17]. Alginate microcapsules have been applied for various purposes, and the molecular cutoff of alginate microcapsule membrane was 75 kDa[18], so the IL-12 protein (a molecular weight of 70 kDa) could pass through the membrane. In the present study, we observed that mIL-12 protein could release freely from the microencapsulated NIH3T3-mIL-12 cells. Twenty-one days after the microencapsulated NIH3T3-mIL-12 cells were transplanted subcutaneously into the tumor-bearing mice, both the NK and CTL activities were significantly enhanced, and the mice serum average concentrations of mIL-12, mIL-2 and mIFN-γ were upregulated, but the mIL-4 and mIL-10 were downregulated in the treated group as compared with those of other control groups. In tumor bearing mice, Th1 cytokine production (IL-2, IFN-γ) is suppressed and Th2 cytokine production (IL-4, IL-10) was increased, as compared with those of normal mice. The administration of microencapsulated NIH3T3-mIL-12 cells to tumor bearing mice transferred the balance of Th1/Th2 cell responses from Th2 dominant state to the Th1 dominant state. These findings are consistent with the results of previous studies showing that production of IFN-γ, NK cell activation, CTL differentiation, and Th1 differentiation were the main mechanisms of antitumor activity of IL-12[19].
The present study developed an alternative approach for local long-term delivery of mIL-12 by a single administration of alginate microcapsules containing cells secreting mIL-12. Using this system, the microencapsulated engineered cells could supply the appropriate doses of effective mIL-12 protein in a paracrine fashion to induce potent anti-tumor immune response and constituted an efficacious therapy in mouse colon models. This system differs from other cytokine gene therapy models, which utilize engineered autologous somatic cells[20,21], tumor cells[22-24] or intratumoral injection of adenovirus expressing cytokine[25,26]. Considering the difficulties of prolonged culture and transduction of human autologous somatic cells or primary tumor cells for each patient, and in contrast, the ready availability of microencapsulated cells, the use of microencapsulated engineered cells for prolonged cytokine administration is an attractive alternate method for clinical application of gene therapy. With respect to the finding that local secretion of IL-12 at the site of tumor might induce an immune response against poorly immunogenic tumor without severe toxicities that were often observed with systemic administration, this system has significant advantages for initiating studies of the prolonged delivery effect of IL-12 with microencapsulated engineered cells on tumor growth.
The transplantation of the microencapsulated NIH3T3-mIL-12 cells could lead to prolonged, homogeneous expression of mIL-12 and continuous stimulation of TILs, with tumor-specific immunity ultimately being established. Such immunity is advantageous because it could result in continued destruction of tumor cells even after expression of mIL-12 had declined[27]. Moreover, no side effects of IL-12 were noticed in treated mice, which is in contrast to the results of trials using recombinant IL-12 protein, where severe toxicity (e.g. fur ruffling or lethargy) was often observed with systemic administration. This is probably because mIL-12 mainly restricted to the vicinity of tumors, a prolonged appropriate blood concentration of cytokines could stimulate an antitumor immune response without causing excessive systemic inflammatory and immunoreaction[28]. Thus this approach should be a better-tolerated and safer strategy than systemic administration of recombinant IL-12 protein. In this study, by treatment of a single dose of microcapsules containing NIH3T3-mIL-12 cells, the growth of tumor xenografts was significantly inhibited and the mice survival time was significantly prolonged. This result also showed that the approach for local and sustained release of interleukin 12 could induces both innate and adaptive antitumor immune responses resulting in significant growth suppression and metastases of tumor[29-31].
It should also be emphasized that controlling the amount of encapsulated cells makes an appropriate concentration of IL-12 obtainable. Preliminary in vitro test for IL-12 expression revealed that the microencapsulated NIH3T3-mIL-12 cells secreted up to 468 ng of mIL-12 per 24hrs per 106 cells. This result indicates that using an optimized amount of encapsulated cells may lead to more powerful antitumor effects and less side effects. Furthermore, using this approach, the antitumor effects of IL-12 may be augmented by combination with other therapeutic genes (e.g., genes encoding other cytokines and apoptotic genes), which is probably necessary in destruction and prevention of recurrence of not only primary tumors, but also metastases[32].
Colorectal carcinomas are generally not very sensitive to the established chemotherapeutic agents and most patients with colorectal carcinoma will die from distant metastases that are not detectable at the initiation of treatment, the alternative antitumor therapy approaches, such as biotherapy, are necessary for the patients suffering from colorectal cancer[33-35]. This study show that microencapsulated engineered cells could supply appropriate doses of effective mIL-12 protein locally to induce potent anti-tumor immune response and constitute an efficacious therapy in mouse colon models. Investigation of optimal combinations of genes used with encapsulated cells has the potential to contribute to a successful anticancer gene therapy for colon cancer.