Published online May 15, 2003. doi: 10.3748/wjg.v9.i5.905
Revised: October 23, 2002
Accepted: January 3, 2003
Published online: May 15, 2003
AIM: To investigate the relationship between inactivation of p16 gene and gastric carcinoma, and the mechanism of inactivation of p16 gene in gastric carcinogenesis.
METHODS: 40 fresh tumor tissue specimens were taken from primary gastric cancer patients. Expression of p16 protein was detected by immunohistochemical method. Deletion and point mutation of p16 gene were analyzed by polymerase chain reaction (PCR) and DNA sequencing, respectively.
RESULTS: The frequency of loss of p16 protein expression in the gastric cancer tissue, adjacent nontumor tissue, and distal normal tissue was 77.5% (31/40), 55.0% (22/40), and 17.5% (7/40), respectively (P < 0.005). Homozygous deletion of exon 1 and exon 3 was observed in two and three cases, respectively, giving an overall frequency of homozygous deletion of 12.5%. All five cases had diffuse type gastric carcinoma. No p16 gene point mutation was detected.
CONCLUSION: These findings suggest a close correlation between inactivation of p16 gene and gastric carcinoma. Further investigations are needed to testify the mechanism of inactivation of p16 gene in gastric carcinogenesis.
- Citation: Zhao GH, Li TC, Shi LH, Xia YB, Lu LM, Huang WB, Sun HL, Zhang YS. Relationship between inactivation of p16 gene and gastric carcinoma. World J Gastroenterol 2003; 9(5): 905-909
- URL: https://www.wjgnet.com/1007-9327/full/v9/i5/905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i5.905
Gastric carcinoma (GC) is common in China[1-19]. From 1991 to 2000, 18, 029 inpatients with malignant tumor had been treated in the Affiliated Yijishan Hospital of Wannan Medical College. 2, 859 cases were GC sufferers, which accounted for 16% of the total inpatients and ranked the first in malignant tumor. The statistical data showed that GC was one of the commonest tumors in South Anhui province. Therefore, it would have clinical significance to explore the pathogenesis of gastric carcinogenesis.
Early in 1994, Kamb et al[20] and Norobi et al[21] reported their studies on p16 gene simultaneously but independently. p16 gene is located in chromosome 9p21, consisting of 2 introns and 3 exons. Exon 1 contains a region of 126 bp, while exon 2 contains a region of 307 bp, and exon 3 contains a region of 11 bp. In recent years, studies have revealed homozygous deletion and mutation of p16 gene, predominantly in exon 2, in various malignant tumor, suggesting that p16 gene is a multiple tumor suppressor 1 (MTS1)[20-33].
The mechanism of p16 gene in gastric carcinogenesis remains unidentified. Some studies have suggested that p16 gene alteration in GC presents itself in different mechanism from other tumors. The alteration is infrequent in exon 2, but exists predominantly in exon 1 and 3[34-36]. In this study, using polymerase chain reaction (PCR), DNA sequencing analysis and an immunohistochemical method, the fresh tumor specimens of 40 GC patients were examined for homozygous deletion, point mutation of p16 gene and expression of p16 protein to verify the relationship between p16 gene alteration and GC.
Under sterile conditions, fresh tumor specimens and their adjacent non-tumor (≤ 3 cm away from the tumor) and distal (≥ 5 cm away from the tumor) normal appearing tissues were obtained in the course of surgery from 40 patients with primary GC at the Affiliated Yijishan Hospital of Wannan Medical College. None of the patients had received either chemotherapy or radiotherapy prior to surgery. All of the primary tumors were pathologically confirmed to be GC cases, among which there were 26 males and 14 females. The age ranged from 31 to 68 (mean 55.4) years old. 14 cases had intestinal type and 26 cases diffuse type by histopathological typing. 12 cases had well-differentiated and 28 cases poorly differentiated.
Approximately 50 mg tissue of GC was abraded with routine methods and digested with proteinase K. DNA was extracted by means of the routine phenol-chloroform method. The purity and concentration of extracted DNA were detected with ultraviolet spectrophotometer (Daojin Company, UV-2201 type). The extracted DNA was stored at 4 °C until use.
The three exons of p16 gene were amplified by employing 3 pairs of primers. The primers 1 and 3 were synthesized by Shanghai Boya Company, and the primer 2 by Saer Biotechnology Company of Shanghai Cell Biology Institution. The sequence of primers and the length of PCR products were listed in Table 1.
| Exon | Sequence of the primer | Product of PCR (bp) | Temperature for annealing (°C) |
| 1S | 5’TCTGCGGAGAGGGGGAGAGCAG3’ | 280 | 58 |
| 1A | 5’GCGCTACCTGATTCCAATTC3’ | ||
| 2S | 5’TTCCTTTCCGTCATGCCGG 3’ | 394 | 56 |
| 2A | 5’GTACAAATTCTCAGATCATCAGTCCTC3’ | ||
| 3S | 5’GGATGTTCCACACATCTTTG3’ | 189 | 52 |
| 3A | 5’ATGAAAACTACGAAAGCGGG3’ |
PCR was performed in 50 μL reaction volume containing 0.2 μg DNA template, 2 U Taq DNA polymerase (Shanghai Sangon Company), 10 pmol/L each pair of primers, 0.2 mmol/L dNTP, 1.5 mmol/L MgCl2, 5 μL 10 × buffer. PCR reaction was carried out in a thermal cycle machine (Perkin-Elmer, 480 type) for an initial 5 min denaturation step at 98 °C. After Taq DNA polymerase was added, the thermal cycle followed. The reaction conditions consisted of denaturation at 94 °C for 45 s, annealing at 58 °C for 50 s for exon 1, 56 °C for 60 s for exon 2, 52 °C for 50 s for exon 3, extension at 72 °C for 60 s, on completion of 35 cycles, at last extension at 72 °C for 5 min. 10 μL of PCR product was loaded into the gel, and a 100 bp DNA ladder was used as a marker. The PCR products were electrophoresed at voltage of 100 V on 2% agarose gel for 30 min and visualized under UV illumination using an ethidium bromide stain. The results were photographed by a digital camera.
The GC specimens that produced amplified product were reamplified by PCR. PCR was performed in 100 μL reaction volume. The products were purified using Spin PCRapid Purification kit (Promage Company), and then together with PCR primers were sequenced (dideoxy chain termination) by an ABI Prism 377 DNA sequencer (Perkin-Elmer Company) in Shanghai Sangon Company.
The sections were deparaffined, boiled and retrieved in citric acid buffer for 10 min. Then, the immunohistochemical streptavid-in-peroxidase (SP) method was used according to the specification of SP kit. The known positive sections were used as a positive control. Phosphatic buffer solution (PBS) replacing the primary antibody was used as a negative control in every experiment. Mouse-anti-human p16 (ZJ11) and SP kit were products of Fuzhou Maixin Biotechnology Company.
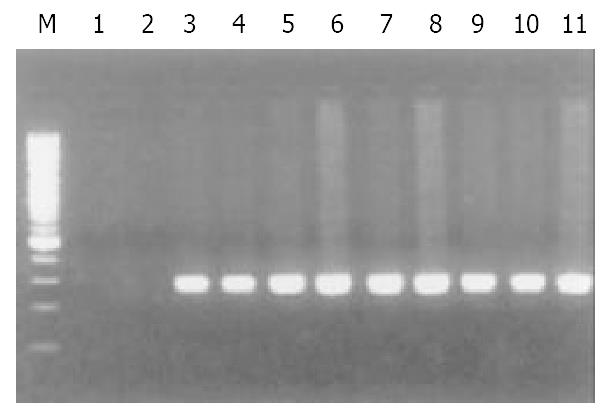
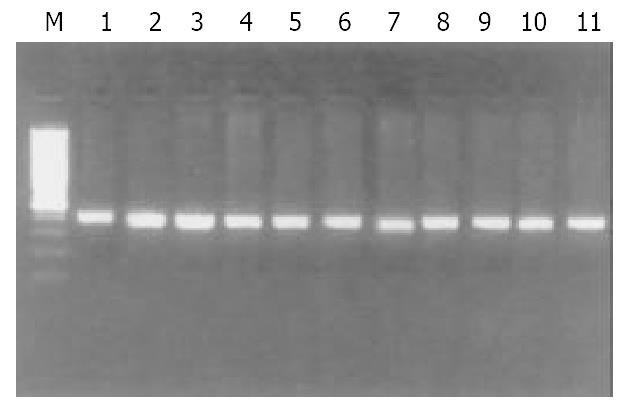
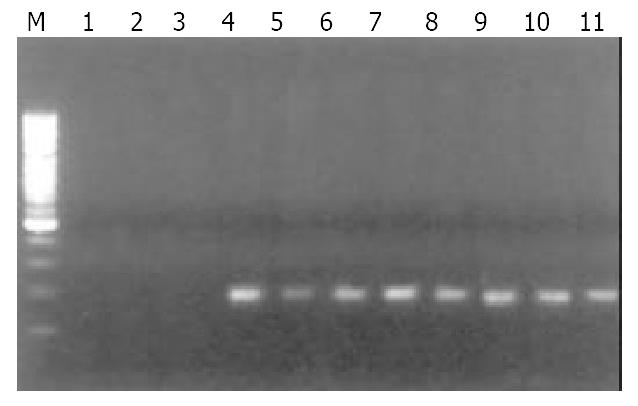
Of 40 GC specimens in PCR products, exon 1 and exon 3 were not detected in 2 cases and 3 cases, respectively. But all of specimens were detected the PCR product of exon 2. These findings indicate that homozygous deletion of p16 gene exon 1 and exon 3 exist in GC (Figure 1, Figure 2, Figure 3). The frequency of deletion of p16 gene was 12.5% (5/40). All five cases with p16 gene deletion had poorly differentiated were all diffuse type GC, with staging of PT3N2M0, as assessed by pathological section analysis.
Amongst the 35 GC cases in whom p16 deletion was not detected, 10 cases were randomly selected for DNA sequencing. No point mutation was detected in these GC specimens when compared with the normal p16 gene cDNA sequence[37].
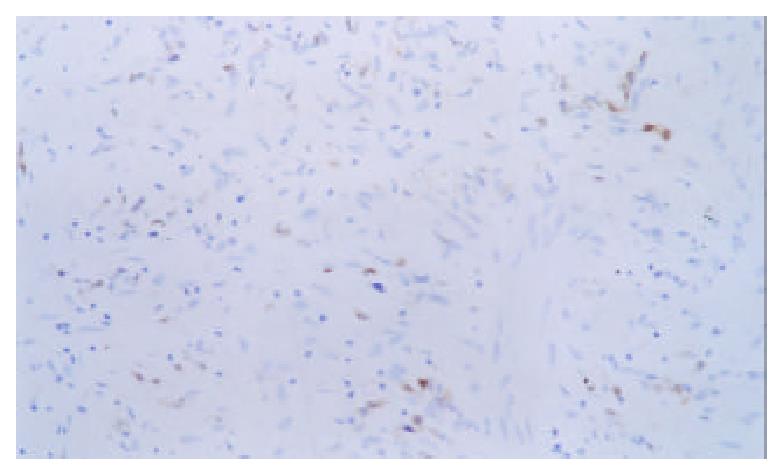
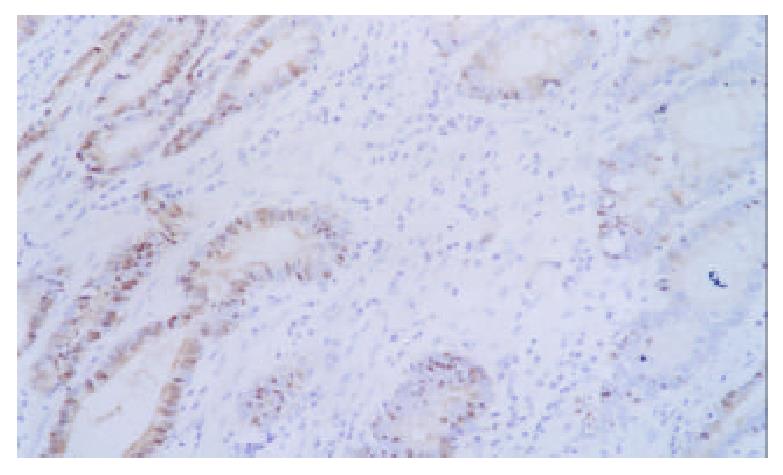
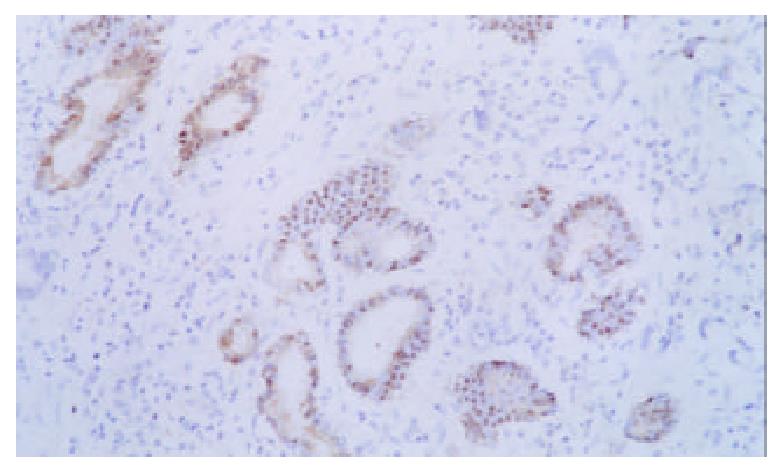
Expression of p16 protein in GC tissues, adjacent nontumor tissue (≤ 3 cm) and distal normal tissue (≥ 5 cm) were summarized in Table 2 and Figure 4, Figure 5, Figure 6. Using χ2 test, the value of χ2 was 29.40, P < 0.005, which has significant difference. These data revealed a strong correlation between inactivation of p16 gene and gastric carcinogenesis.
| Hisological types | n | Positive | Negative | The frequency of p16 protein expression loss (%) |
| Gastric carcinoma | 40 | 9 | 31 | 77.5 |
| Adjacent nontumor tissue ( ≤ 3 cm) | 40 | 18 | 22 | 55.0 |
| Distal normal tissue (≥ 5 cm) | 40 | 33 | 7 | 17.5 |
Fission of tumor cells undergoes a regular cell cycle as normal cells do, which is commonly divided into G1, S, G2, and M phase. When a cell goes into the cell cycle, the cell depends on activation of cyclin dependent kinase (CDK). Only combined with a cyclin, can CDK be activated, and inhibited when it combines with a series of proteins. These proteins known as cyclin dependent kinase inhibitors are a group of small molecular proteins that are involved in the negative regulation of the cell cycle. Alteration of these proteins results in uncontrollable cell proliferation and thus carcinogenesis. p16 protein is one of these proteins so that mutation and deletion of p16 gene lead to uncontrollable cell proliferation, and then carcinogenesis[1,20,21,37-40]. The relationship between alteration of p16 gene and carcinogenesis of various tumors has been verified[1,20-36,41].
In this study, homozygous deletion of exon 1 and exon 3 was observed, respectively, in 2 cases and 3 cases with poorly differentiated diffuse type GC amongst 40 samples. The frequency of deletion was 12.5%. Our results showed that alteration in p16 gene was highly associated with poorly differentiated GC, which is almost identical to the observations by Lu et al[34] and Lee et al[35]. Therefore, it is suggested that alteration of p16 gene is associated with the differentiation degree and metastasis of GC. In contrast, Wu et al[36] reported that deletion of p16 gene was frequently encountered in the intestinal type, with a lower frequency in the diffuse type. Furthermore, He et al[41] reported that there was homozygous deletion of p16 gene exon 2 in GC, with a frequency of 20.0%. Our finding does not tally with above observation[34-36]. We randomly selected 10 of the 35 GC cases without p16 gene deletion for DNA sequencing, no point mutation was detected. The results reveal that point mutation of p16 gene is rare in gastric carcinogenesis. It coincides with previous findings[34-36,41].
In human primary GC, deletion and point mutation of p16 gene are infrequent, but loss of expression of p16 protein is common. Some studies have found[1,35,40-42] that the frequency of loss of p16 protein expression ranges from 52% to 90%. Our results agree with these studies. We found that the frequency of loss of expression of p16 protein was 77.5%. It had statistically significant difference with adjacent non-tumor tissue (55.0%) and distal normal appearing tissue (17.5%) (P < 0.005). These results suggest that inactivation of p16 gene is strongly associated with gastric caicinogenesis, however, deletion and point mutation of p16 gene are not the major mechanism of p16 inactivation in GC.
The methylation-specific PCR (MSP) method established by Herman et al[43] in 1996 has significantly promoted the research on the interrelationship between hypermethylation and gene silencing. Subsequent studies have revealed high frequency of hypermethylation in p16 gene 5’ promotor regions in various tumors, with frequency ranging from 60% to 89%[44-48]. Inactivation of p16 gene correlates with hypermethylation of 5’ promotor regions CpG island in GC. Some studies have found that hypermethylaion in the promotor regions CpG island is an important mechanism for p16 gene inactivation in GC, whereas deletion and point mutation of the gene are rarely seen[49-56]. The CpG island hypermethylation occurred early in multistep gastric carcinogenesis tends to accumulate along the process[55]. p16 gene with methylated promotor regions would reexpress p16 protein when it is treated with 5-aza-2’-deoxycytidine which demethylates[35] or when the inducers of hypermethylation are eliminated[57,58]. The effect mechanism of p16 gene is to inhibit cell cycle directly, and it is easy to perform gene targeting or protein modification because p16 gene is small. Therefore, it is needed to further investigate the relationship between p16 gene inactivation and gastric carcinogenesis, and the mechanism of p16 gene inactivation, since it is of significant clinical implications in early diagnosis, therapy and prognosis.
Edited by Xia HHX
| 1. | Zhao Y, Zhang XY, Shi XJ, Hu PZ, Zhang CS, Ma FC. Clinical significance of expressions of P16, P53 proteins and PCNA in gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:246-248. |
| 2. | Tu SP, Jiang SH, Tan JH, Jiang XH, Qiao MM, Zhang YP, Wu YL, Wu YX. Proliferation inhibition and apoptosis induction by ar-senic trioxide on gastric cancer cell SGC-7901. Shijie Huaren Xiaohua Zazhi. 1999;7:18-21. |
| 3. | Gao P, Jiang XW, Yuan WJ. Effects of gastrin and gastrin receptor antagonist proglumide on gastric cancer line. Shijie Huaren Xiaohua Zazhi. 1999;7:22-24. |
| 4. | Li JQ, Wan YL, Cai WY. Biological significance of cyclin E expression in early gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:31-33. |
| 5. | Dong WG, Yu JP, Luo HS, Yu BP, Xu Y. Relationship between human papillomavirus infection and the development of gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:46-48. |
| 6. | Liu ZM, Shou NH. Significance of mdr1 gene expression in gastric carcinoma tissue. Shijie Huaren Xiaohua Zazhi. 1999;7:145-146. |
| 7. | Shi YQ, Xiao B, Miao JY, Zhao YQ, You H, Fan DM. Construction of eukaryotic expression vector pBK fas and MDR reversal test of drug-resistant gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:309-312. |
| 8. | Fang DC, Zhou XD, Luo YH, Wang DX, Lu R, Yang SM, Liu WW. Microsatellite instability and loss of heterozygosity of suppressor gene in gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:479-481. |
| 9. | Qin LJ. In situ hybridization of P53 tumor suppressor gene in human gastric precancerous lesions and gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:494-497. |
| 10. | Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its precancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649-651. |
| 11. | Mi JQ, Zhang ZH, Shen MC. Significance of CD44v6 protein expression in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:156-158. |
| 12. | Gao GL, Yang Y, Yang S, Ren CW. Relationship between proliferation of vascular andothelial cells and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:282-284. |
| 13. | Wang RQ, Fang DC, Liu WW. MUC2 gene expression in gastric cancer and preneoplastic lesion tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:285-288. |
| 14. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. Bax gene expression and its relationship with apoptosis in human gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:665-668. |
| 15. | Gu HP, Ni CR, Zhan RZ. Relationship between CD15 mRNA and its protein expression and gastric carcinoma invasion. Shijie Huaren Xiaohua Zazhi. 2000;8:851-854. |
| 16. | Wang DX, Fang DC, Liu WW. Study on alteration of multiple genes in intestinal metaplasia, atypical hyperplasia and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:855-859. |
| 17. | Guo SY, Gu QL, Liu BY, Zhu ZG, Yin HR, Lin YZ. Experimental study on the treatment of gastric cancer by TK gene combined with mIL-2 gene. Shijie Huaren Xiaohua Zazhi. 2000;8:974-978. |
| 18. | Guo YQ, Zhu ZH, Li JF. Flow cytometric analysis of apoptosis and proliferation in gastric cancer and precancerous lesion. Shijie Huaren Xiaohua Zazhi. 2000;8:983-987. |
| 19. | Xia JZ, Zhu ZG, Liu BY, Yan M, Yin HR. Significance of immunohistochemically demonstrated micrometastases to lymph nodes in gastric carcinomas. Shijie Huaren Xiaohua Zazhi. 2000;8:1113-1116. |
| 20. | Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, Johnson BE, Skolnick MH. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 1888] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 21. | Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1166] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 22. | Lu JG, Huang ZQ, Wu JS, Wang Q, Ma QJ, Yao X. Significance of tumor suppressor gene p16 expression in primary biliary cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:638-640. |
| 23. | Zhou YA, Gu ZP, Wang XX, Ma QF, Huang LJ. Reexpression of p16INK4a gene suppresses growth of human esophageal cercinoma cells. Shijie Huaren Xiaohua Zazhi. 2001;9:877-881. |
| 24. | Jin S, Peng Q, Lu S. [Deletion of MTS1/p16 gene in human esophageal carcinoma]. Zhonghua Zhongliu Zazhi. 1998;20:9-11. [PubMed] |
| 25. | Peng Q, Jin S, Lu S. [p16 gene suppresses growth of human esophageal carcinoma cells]. Zhonghua Zhongliu Zazhi. 1999;21:175-177. [PubMed] |
| 26. | Lu G, Chen X, Cao W, Zhang G, Dai H, Jiang Y, Sun W. Multiple tumor suppressor 1/p16 gene alterations in human esophageal squamous-cell carcinoma: clinical significance and regional difference. Zhonghua Zhongliu Zazhi. 1999;21:359-362. |
| 27. | Cheng J, Lin C, Xing R. [Apoptosis of human melanoma cell line WM-983A by p16 gene transduction]. Zhonghua Zhongliu Zazhi. 1999;21:89-92. [PubMed] |
| 28. | Hashemi J, Platz A, Ueno T, Stierner U, Ringborg U, Hansson J. CDKN2A germ-line mutations in individuals with multiple cutaneous melanomas. Cancer Res. 2000;60:6864-6867. [PubMed] |
| 29. | Wang X, Sun Y, Li W. [Alterations of FHIT gene and p16 gene in lung cancer and metastatic hilar lymph nodes]. Zhonghua Zhongliu Zazhi. 1999;21:108-111. [PubMed] |
| 30. | Fu X, Zhang S, Ran R. [Effect of exogenous p16 gene on the growth of wild-type p53 human lung adenocarcinoma cells]. Zhonghua Zhongliu Zazhi. 1999;21:102-104. [PubMed] |
| 31. | Zhou J, Yang D, Zhang L, Wang J, Yao Q, Su Z, Fan Y. [Study on the relationship of alteration and expression of p16 gene to pancreatic carcinoma]. Zhonghua Yixue Yichuanxue Zazhi. 2000;17:399-403. [PubMed] |
| 32. | Wang GL, Lo KW, Tsang KS, Chung NY, Tsang YS, Cheung ST, Lee JC, Huang DP. Inhibiting tumorigenic potential by restoration of p16 in nasopharyngeal carcinoma. Br J Cancer. 1999;81:1122-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Huang Q, Tao Y, Yandell DW. [Mutations of several tumor suppressor genes in primary retinoblastoma]. Zhonghua Zhongliu Zazhi. 1999;21:10-12. [PubMed] |
| 34. | Lu Y, Gao C, Cui J. [Deletion and down-regulation of mts1/p16 gene in human gastric cancer]. Zhonghua Zhongliu Zazhi. 1996;18:189-191. [PubMed] |
| 35. | Lee YY, Kang SH, Seo JY, Jung CW, Lee KU, Choe KJ, Kim BK, Kim NK, Koeffler HP, Bang YJ. Alterations of p16INK4A and p15INK4B genes in gastric carcinomas. Cancer. 1997;80:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 36. | Wu MS, Shun CT, Sheu JC, Wang HP, Wang JT, Lee WJ, Chen CJ, Wang TH, Lin JT. Overexpression of mutant p53 and c-erbB-2 proteins and mutations of the p15 and p16 genes in human gastric carcinoma: with respect to histological subtypes and stages. J Gastroenterol Hepatol. 1998;13:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704-707. [PubMed] |
| 38. | Shapiro GI, Edwards CD, Ewen ME, Rollins BJ. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol Cell Biol. 1998;18:378-387. [PubMed] |
| 39. | Quan J, Fan XG. Progress in experimental research of Helicobacter pylori infiction and gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:1068-1069. |
| 40. | Wu SH, Ma LP, Jin W, Sui YF. The relation between suppressor gene p16, p21, PRB and gastric cancer. Shilie Huaren Xiaohua Zazhi. 1999;7:551. |
| 41. | He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515-521. [PubMed] |
| 42. | Zhou Y, Gao SS, Li YX, Fan ZM, Zhao X, Qi YJ, Wei JP, Zou JX, Liu G, Jiao LH. Tumor suppressor gene p16 and Rb expression in gastric cardia precancerous lesions from subjects at a high incidence area in northern China. World J Gastroenterol. 2002;8:423-425. [PubMed] |
| 43. | Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821-9826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4183] [Cited by in RCA: 4248] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 44. | Lehmann U, Hasemeier B, Lilischkis R, Kreipe H. Quantitative analysis of promoter hypermethylation in laser-microdissected archival specimens. Lab Invest. 2001;81:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Matsuda Y, Ichida T, Matsuzawa J, Sugimura K, Asakura H. p16(INK4) is inactivated by extensive CpG methylation in human hepatocellular carcinoma. Gastroenterology. 1999;116:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Liew CT, Li HM, Lo KW, Leow CK, Chan JY, Hin LY, Lau WY, Lai PB, Lim BK, Huang J. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene. 1999;18:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Foster SA, Wong DJ, Barrett MT, Galloway DA. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol Cell Biol. 1998;18:1793-1801. [PubMed] |
| 48. | Salem C, Liang G, Tsai YC, Coulter J, Knowles MA, Feng AC, Groshen S, Nichols PW, Jones PA. Progressive increases in de novo methylation of CpG islands in bladder cancer. Cancer Res. 2000;60:2473-2476. [PubMed] |
| 49. | Suzuki H, Itoh F, Toyota M, Kikuchi T, Kakiuchi H, Hinoda Y, Imai K. Distinct methylation pattern and microsatellite instability in sporadic gastric cancer. Int J Cancer. 1999;83:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, Baylin SB, Issa JP. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 1999;59:5438-5442. [PubMed] |
| 51. | Tsujie M, Yamamoto H, Tomita N, Sugita Y, Ohue M, Sakita I, Tamaki Y, Sekimoto M, Doki Y, Inoue M. Expression of tumor suppressor gene p16(INK4) products in primary gastric cancer. Oncology. 2000;58:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Shim YH, Kang GH, Ro JY. Correlation of p16 hypermethylation with p16 protein loss in sporadic gastric carcinomas. Lab Invest. 2000;80:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Song SH, Jong HS, Choi HH, Kang SH, Ryu MH, Kim NK, Kim WH, Bang YJ. Methylation of specific CpG sites in the promoter region could significantly down-regulate p16(INK4a) expression in gastric adenocarcinoma. Int J Cancer. 2000;87:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Leung WK, Yu J, Ng EK, To KF, Ma PK, Lee TL, Go MY, Chung SC, Sung JJ. Concurrent hypermethylation of multiple tumor-related genes in gastric carcinoma and adjacent normal tissues. Cancer. 2001;91:2294-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 55. | Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001;61:2847-2851. [PubMed] |
| 56. | Bae SI, Lee HS, Kim SH, Kim WH. Inactivation of O6-methylguanine-DNA methyltransferase by promoter CpG island hypermethylation in gastric cancers. Br J Cancer. 2002;86:1888-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Toyota M, Itoh F, Imai K. Can DNA methylation be a molecular marker for eradication of Helicobacter pylori in MALT lymphoma. J Gastroenterol. 2002;37:73-74. [PubMed] |
| 58. | Kim YS, Kim JS, Jung HC, Lee CH, Kim CW, Song IS, Kim CY. Regression of low-grade gastric mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori: possible association with p16 hypermethylation. J Gastroenterol. 2002;37:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |