Published online May 15, 2003. doi: 10.3748/wjg.v9.i5.1034
Revised: December 23, 2002
Accepted: January 8, 2003
Published online: May 15, 2003
AIM: To determine whether serum leptin level and the leptin receptor (OB-R) expression in the basolateral amygdala (BLA) change following conditioned taste aversion (CTA) formation.
METHODS: The serum leptin concentration was measured by rat leptin RIA kit, long and short forms of leptin receptor (OB-Rb and OB-Ra) mRNA in the brain sections were examined by in situ hybridization (ISH) and the expression of OB-R was assessed by immunohistochemistry ABC method with a highly specific goat anti-OB-R antibody.
RESULTS: The level of serum leptin didn’t show significant difference between CTA and control group. Comparing with the control group, the CTA group had an increase on count of OB-R immunohistochemistry positive-stained cells in the BLA (127 ± 12 vs 48 ± 9 per 1 mm2). The OB-Rb mRNA expression level enhanced by 11.9% in the BLA, while OB-Ra mRNA level increased by 7.4% on the choroid plexus in CTA group. So BLA was supposed to be a region where interactions between gustatory and vagal signals take place.
CONCLUSION: BLA is one of the sites, which are responsible for CTA formation in the brain. Leptin and OB-R maybe involved in neuronal communication for CTA. So leptin and its receptors probably take part in CTA and integration of autonomic and extroceptive information.
- Citation: Han Z, Yan JQ, Luo GG, Liu Y, Wang YL. Leptin receptor expression in the basolateral nucleus of amygdala of conditioned taste aversion rats. World J Gastroenterol 2003; 9(5): 1034-1037
- URL: https://www.wjgnet.com/1007-9327/full/v9/i5/1034.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i5.1034
Conditioned taste aversion (CTA) is a protective reflex by which an animal learns to discriminate and to reject potentially harmful substances by their flavor. When the consumption of novel flavored food is followed by internal malaise, animals avoid ingesting the food on subsequent presentations[1,2]. The anatomical substrates responsible for CTA learning have been well established[3]. But the neurotransmitters involved in neuronal communication for CTA are not yet well known.
Leptin has been discovered since 1994 by Zhang et al[27] (Nature 1994; 372:425-431) as the product of the ob gene. Leptin (OB protein) is produced primarily by adipose tissue and secreted into the bloodstream, then delivered to the brain. In the brain some specific receptors of leptin have been characterized, especially in hypothalamic nuclei where express neuropeptides and neurotransmitters that were involved in the long-term regulation of food intake and metabolism rate[4-7], therefore, leptin serves as a unique feedback signal system to submit information regarding to adipose tissue energy store into the central nervous system[8]. As leptin level growth coincidence with adiposity increment in rodents and mankind, it is proposed to act as a negative feedback ‘adipostatic signal’ to brain centers and control energy homeostasis, prevent from obesity in time of nutritional abundance[9].
In recent years leptin receptor has been found to be expressed in the amygdala[10], especially in the basolateral amygdala (BLA)[11]. The interest in amygdala which acts as a regulator of weight and intake behavior has also been warming up. Amygdala is one of critical centers to regulate weight and ingestive behavior. Robust increases in body weight and food intake has been observed in rats with lesions of the BLA and postorodorsal amygdala (PDA) (Brain Res 1996;740:193-200). In addition to food intake alteration, lesions (electrolytic and excitotoxic) in the amygdala have also been known to disrupt the formation of conditioned taste aversion (CTA), particularly lesions placed in the BLA[12-15]. The sense of taste lies in the interface between the external and internal of milieus and participates in control of motivational processes which guide dietary selection. The amygdala plays an important role in the initiation and guidance of autonomic and exteroceptive information[16].
The present study was undertaken to further investigate possible changes of the leptin receptor expression accompanied with learning and maintaining of CTA.
Twenty healthy adult male Sprague-Dawlay rats, weighing 200-250 g, were used in all experiments. Every rat was housed at one cage in a temperature-controlled room (18-24 °C) on a 12 h light/dark cycle and allowed food (rodent chow) and water ad libitum for 3 days. All rats were randomly grouped into 2 groups. Each group consisted of 10 rats.
Rats were water-deprived for 24 h prior to test day. At 08:30 to 09:00 from day 1 to 5, the rats were trained to drink distilled water for 30 min in the home cages. At the same time on day 6, each rat in test group received an ip injection of 0.15 mol/L LiCl (2% body weight) as an unconditioned stimulus (US) soon after 30 min of free access to 1 g/L (0.005 mol/L) sodium saccharin instead of water as a conditioned stimulus (CS). Rats in control group received an ip injection of an equivalent volume of 0.15 mol/L NaCl. From day 7 to 9, all rats have accessed to distilled water for 30 min. On day 10, the rats were presented with 1 g/L saccharin for 30 min. The volumes of intake of the CS were recorded on day 6 and 10.
Rats were anaesthetized with urethane (1.2 g/kg, ip). Then the tail was cut, the whole blood glucose level was measured by One Touch Brand blood glucose meter (Lifescan inc, USA). Then opened thorax and 2 mL blood was drawn from the aorta and put into dry tube allowing to clot for 1 h. Serum was separated by centrifugation (2000 r/min) for 20 min. Then the rats were perfused via the ascending aorta with 300 ml of 8.5 g/L saline (room temperature) followed by 300 mL of paraformaldehyde (PFA, 4 g/L, pH7.4). Brains were dissected out and then post-fixed in the same fixative for 3 hours at 4 °C. After being paraffin embedded, three series of sections were cut coronally at a thickness of 5 μm, thaw-mounted on poly-L-lysme-coated glass slides and stored at -70 °C. One of the series was used for immunohistochemical staining, another for ISH. The remaining series of sections were stained with hematoxylin-eosin.
Serum leptin and insulin were measured by radioimmunoassay (RIA) (rat leptin RIA kit, rat insulin RIA kit, Linco Research, St, Louis, MO). The lower limits of sensitivity of the assay are 0.5 µg/L and 0.1 µg/L.
Endogenous peroxidase activity was blocked with 0.01 mol/L PB containing 0.3 mL/L hydrogen peroxide. Then the sections were heated to 92 °C in citric acid (pH6.0). After that the sections were rinsed with 0.01 mol/L phosphate-buffered saline (PBS), incubated with 15 mL/L blocking serum in PBS for 1 h. The sections were incubated at 4 °C for 40 h with goat polyclonal antiserum against leptin receptor (antiserum Sc-1834 with dilution at 1:100, Santa-Cruz Biotechnology, CA, USA). After incubation, the sections were immunostained by the avidin-biotin complex method (goat ABC staining system, Sc-2023, Santa-Cruz Biotechnology, CA, USA). The sections were mounted on slide glasses, dried and dehydrated in a graded ethanol series, and covered with balata.
To verify the specificity of staining, some sections were incubated with non-immune serum instead of the primary antiserum, or with the primary antiserum without the second antiserum to serve as a negative control. No LR-IR was observed in the negative control tissue.
Prior to hybridization, the sections were deparaffinized, incubated at 37 °C in 2 mg/L of proteinase K for 25 min. Post fixation was performed in a PFA solution and the sections were treated with 2 mol/L HCl. The specific oligonucleotide probes are 5’-GGCTCCAGAAGAAGACCAAATATC (Nucleotide number 2712-2738 of Genebank sequence D84500) for OB-Rb and 5’-CAAGCATGGGCTGCAGTGACATTAGAG (Nucleotide number 671-697 of Genebank sequence D84550) for OB-Ra. The probe was terminally labeled with digoxigenin-dUTP (Dig Oligonucleotide Tailing Kit, Cat No. 1417231, Roche, Germany) and tested by Dig nucleic acid detection kit (1175041, Roche, Germany). Its concentration was 100 nmol/L. Probe was prepared in a solution containing 500 g/L formamide, 0.3 mol/L NaCl, 10 mmol/L Tris, 1 g/L ssDNA, 1 × Denhardt’s solution, and 100 g/L dextran sulfate, then they were hybridized to sections at 50 °C for 16-18 h. After hybridization, the slides were washed sequentially in 2 × saline-sodium citrate (SSC) for 30 min followed by rinses in 1 × SSC, 0.5 × SSC. Then they were incubated with phosphatase-labeled anti-digoxigenin antibody (1:400) at 37 °C for 2 h. Staining was performed with a freshly prepared substrate solution of Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. Then the sections were restained by methyl green.
To assess the specificity of labelling, control sections were co-incubated with a 100-fold excess of unlabelled probe in addition to the corresponding DIG-labelled probe. No labelling above background was detected in those sections.
On the basis of volumes of following two test days, we calculated a CTA index as an indicator of the strength of CTA formation. The larger this index, the stronger the acquisition of CTA: CTA index = 1 - (total saccharin intake on day 10/total saccharin intake on day 6).
Some sections were photographed. Then expression of leptin receptor immunoreactivity was quantified by counting positive-stained cells in BLA of five adjacent brain sections. All films of ISH were analyzed by using a computer-assisted image analysis system, multi-analyst, connected to a GS 690 Imaging Densitometer (Bio-Rad, USA). Quantification of mRNA expression levels was obtained by measuring the average density of each region in five adjacent brain sections. All data are shown as the -x±sx for groups based on eight rats in each group. Analysis was performed by SPSS software. Unpaired, two tailed t-test and Anova comparison test were used with P < 0.05 be considered as sufficient to reject no difference hypothesis.
There were no significant differences of weight, glucose level, serum leptin and serum insulin level (Compared with control rats, P > 0.05) between test and control groups (Table 1).
| Group | n | Weight (g) | Glucose | Leptin (µg/L) | Insulin(µg/L) |
| CTA | 8 | 227.2 ± 11.7 | 6.2 ± 0.8 | 2.2 ± 0.4 | 1.2 ± 0.4 |
| Control | 9 | 226.3 ± 10.2 | 6.4 ± 1.2 | 2.1 ± 0.4 | 1.4 ± 0.5 |
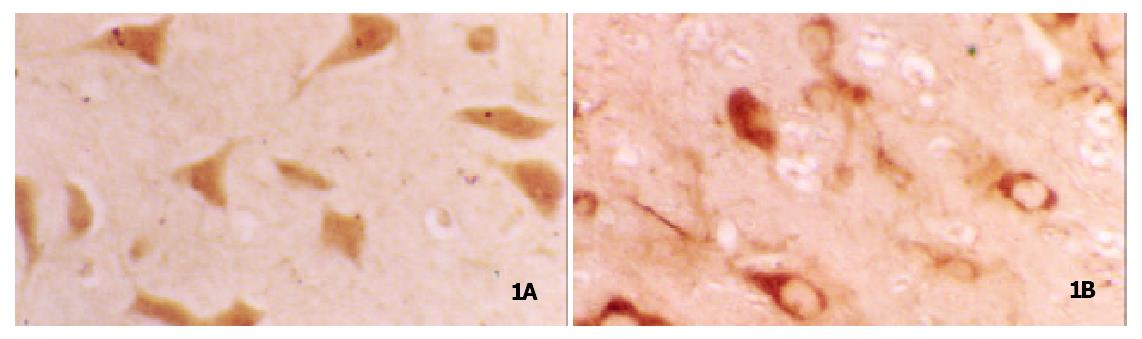
Many neuronal cell bodies and dendritic processes in the amygdala expressed leptin receptor immunoreactivity (LR-IR). The expressed immunoreactivity was quantified by counting positive-stained cells. Compared with the control group, the expression of leptin receptor was increased by 166.7% in BLA. The average number of LR-IR positive cell was 127 ± 12 vs 48 ± 9 per 1 mm2 (t = 12.67, P = 0.000). LR-IR was mainly found in the membrane of cells in the control group. In CTA rats, it was shown that LR-IR mostly deposited in cytoplasm and membrane. That is to say, LR-IR immigrated into cells after CTA leaning (Figure 1).
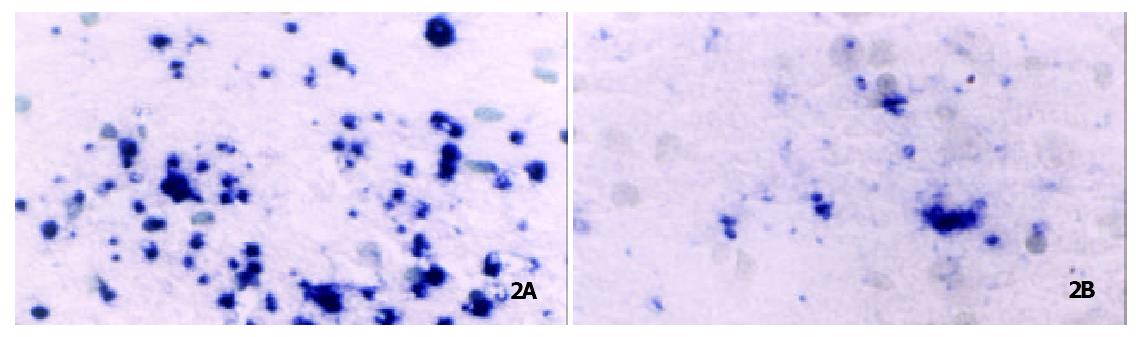
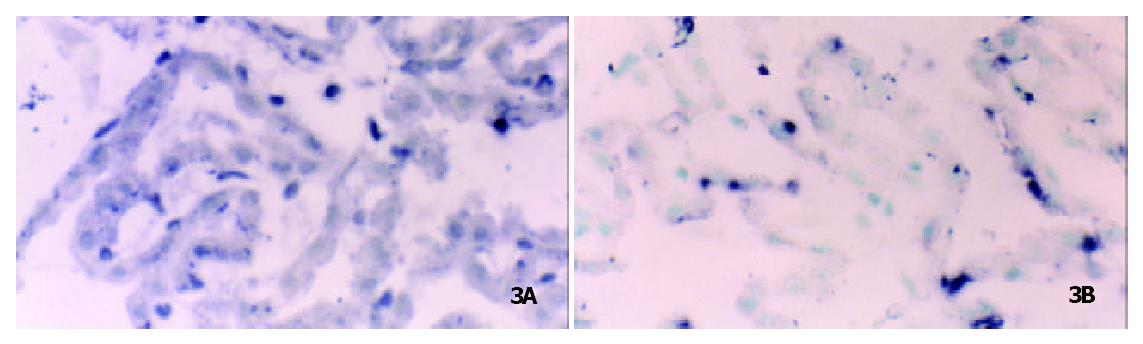
By means of CTA learning, rats displayed a significant difference at the level of OB-Rb mRNA expression in the BLA. Compared with the control group, the levels of OB-Rb mRNA increased by + 11.9% (116.5 ± 10.8 vs 104.3 ± 10.9). The level of OB-Ra mRNA on the choroid plexus also rose up by 7.4% (114.2 ± 12.0 vs 106.1 ± 13.8 (Figure 2, Figure 3)). After CTA learning, the expression of both OB-Rb mRNA in the BLA and Ob-Ra mRNA on the choriod plexus have significantly enhanced.
Many researches have observed deficits in the CTA paradigm after functional disruption of the BLA, which included electrolytic lesions, ibotenic acid lesions[17-20]. The amygdala has long been believed to be associated with the control of emotions, motivation, and hedonic tone[21,22]. The amygdala receives information from various sensory modalities via the neocortex and directly from the thalamus, brain stem and plays an important role in ingestive behaviors. In rodents, the amygdala is one of the major recipients of gustatory projections to forebrain. The nucleus tractus solitarius (NTS) in the medulla receives taste information from peripheral taste nerves and sends gustatory information to the pontine parabrachial nucleus (PBN). Gustatory neurons in the PBN pass through two parallel ascending paths, one path goes to thalamocortical axis and the other directly goes to the amygdala[23]. Gustatory and visceral information from the PBN may travel through the insular cortex or the thalamus, ultimately reaching the BLA. All these appear to be the routes relevant to CTA[2]. On the basis of the typical CTA paradigm in which ingestion of a taste solution is paired with an ip injection of LiCl as an illness-inducing agent, CTA can be thought in a simple framework of association learning between taste information via the taste nerves and general visceral information via the vagus nerve.
So BLA has been characterized as a region where presents of the internal and external worlds overlap, and it permits the animal to assess physiological needs in relation to the external resources, which are available to fulfill. As a result, the amygdala plays a critical role in the initiation and guidance of feeding, on which relies an integration of autonomic and exteroceptive information. Nishijo’s study suggested that the activity of the amygdaloid neurons was altered when animals must modulate ingestive behavior by learning a new stimulus associated with food and being exposed to stress. The amygdala may not contribute to gustatory processing by precise discrimination, but by imparting hedonic appreciation and emotional significance to the taste experience, and by mediating the effects of conditional and of physiological needs on taste perception[24].
Leptin is a hormone believed to control appetite and regulate body weight via receptors[25]. The leptin receptor is widely distributed in the brain, including hypothalamus[26], pituitary[27] and amygdala[10]. These observations suggest that leptin maybe involved not only in the control of energy expenditure, but also in other neuroendocrine functions[28,29]. The expression of OB-Rb and OB-Ra mRNA increased following by CTA formation indicate that leptin and its receptors may take part in CTA learning and interactions between gustatory and vagal signals.
To date, six different alternatively spliced isoforms have been identified, referred to as OB-Ra-f (Nature 1996; 379: 632-635). They are classified by the length of intracellular domains of the receptors. The long form of the receptor, OB-Rb is taken as the main functional receptor, which is capable of signaling, and is thus able to mediate the biological effects of leptin[30]. Short forms, especially OB-Ra may play a role not only in transport but also in clearance or as a source of soluble receptor (J Biol Chem 1997; 272: 6093-6096). The leptin receptor on the choroid plexus plays an important role in transporting plasma leptin into the brain[31].
In the present study, although the level of serum leptin has not changed, strong expression of OB-R protein and OB-Rb mRNA has been found in the BLA and OB-Ra mRNA level increasing on the choroid plexus after CTA formation. All these results suggest that leptin receptor may participate in the formation of CTA. After CTA formation, LR-IR was expressed in the cytoplasm and the membrane of cells. It can be predicted that the function or activity of leptin receptor maybe change in the formation and maintenance of CTA.
Leptin is well known for its regulation of food intake and body weight. It also acts as a sweet-sensing suppressor. After i.p. injection of recombinant leptin, the sucrose and saccharin responses decreased parallelly along with the serum leptin level increased[4]. So leptin and its receptors possibly act as a mediate factor between feeding and taste. It maybe a sweet-sensing modulator that takes part in regulation of food intake. But in the present immunohistochemistry staining, the primary antibody reacts with all isoforms, including the long and short forms of receptors. Thus the antiserum could not discriminate the isoforms. Further studies are necessary to clarify the functional significance of OB-R isoforms of different aspects in taste aversion learning.
In summary, we have discovered (1) Expression of OB-Rb mRNA and OB-R protein were increased by means of CTA learning in the BLA; (2) OB-Ra mRNA levels enchanced followed by CTA formation on the choroid plexus; (3) LR-IR immigrated into cells after CTA formation.
Mrs. Song and Mr. Lai for their technical assistance.
Edited by Pang LH
| 1. | Yasoshima Y, Morimoto T, Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Res. 2000;869:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Welzl H, D'Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res. 2001;125:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Sakai N, Yamamoto T. Possible routes of visceral information in the rat brain in formation of conditioned taste aversion. Neurosci Res. 1999;35:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA. 2000;97:11044-11049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 236] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Scott TR, Verhagen JV. Taste as a factor in the management of nutrition. Nutrition. 2000;16:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Hofbauer KG. Molecular pathways to obesity. Int J Obes Relat Metab Disord. 2002;26 Suppl 2:S18-S27. [PubMed] |
| 7. | Strubbe JH, van Dijk G. The temporal organization of ingestive behaviour and its interaction with regulation of energy balance. Neurosci Biobehav Rev. 2002;26:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Tschöp M, Morrison KM. Weight loss at high altitude. Adv Exp Med Biol. 2001;502:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Blundell JE, Goodson S, Halford JC. Regulation of appetite: role of leptin in signalling systems for drive and satiety. Int J Obes Relat Metab Disord. 2001;25 Suppl 1:S29-S34. [PubMed] |
| 10. | Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Lundy RF, Norgren R. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol. 2001;85:770-783. [PubMed] |
| 12. | Miranda MI, Ferreira G, Ramirez-Lugo L, Bermudez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proc Natl Acad Sci USA. 2002;99:11417-11422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Gutiérrez H, Gutiérrez R, Ramírez-Trejo L, Silva-Gandarias R, Ormsby CE, Miranda MI, Bermúdez-Rattoni F. Redundant basal forebrain modulation in taste aversion memory formation. J Neurosci. 1999;19:7661-7669. [PubMed] |
| 14. | Wig GS, Barnes SJ, Pinel JP. Conditioning of a flavor aversion in rats by amygdala kindling. Behav Neurosci. 2002;116:347-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Ganaraja B, Jeganathan PS. Increased sweet taste preference following the lesion of basolateral nucleus of amygdala (BLA) in rat. Indian J Physiol Pharmacol. 1999;43:443-448. [PubMed] |
| 16. | Ganaraja B, Jeganathan PS. Effect of basolateral amygdala & amp; ventromedial hypothalamic lesions on ingestion & amp; taste preference in rat. Indian J Med Res. 2000;112:65-70. [PubMed] |
| 17. | Ferry B, Di Scala G. Basolateral amygdala NMDA receptors are selectively involved in the acquisition of taste-potentiated odor aversion in the rat. Behav Neurosci. 2000;114:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Rollins BL, King BM. Amygdala-lesion obesity: what is the role of the various amygdaloid nuclei? Am J Physiol Regul Integr Comp Physiol. 2000;279:R1348-R1356. [PubMed] |
| 19. | Rollins BL, Stines SG, McGuire HB, King BM. Effects of amygdala lesions on body weight, conditioned taste aversion, and neophobia. Physiol Behav. 2001;72:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Morris R, Frey S, Kasambira T, Petrides M. Ibotenic acid lesions of the basolateral, but not the central, amygdala interfere with conditioned taste aversion: evidence from a combined behavioral and anatomical tract-tracing investigation. Behav Neurosci. 1999;113:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res. 2002;136:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Nishijo H, Uwano T, Tamura R, Ono T. Gustatory and multimodal neuronal responses in the amygdala during licking and discrimination of sensory stimuli in awake rats. J Neurophysiol. 1998;79:21-36. [PubMed] |
| 23. | Pritchard TC, Hamilton RB, Norgren R. Projections of the parabrachial nucleus in the old world monkey. Exp Neurol. 2000;165:101-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Nishijo H, Ono T, Uwano T, Kondoh T, Torii K. Hypothalamic and amygdalar neuronal responses to various tastant solutions during ingestive behavior in rats. J Nutr. 2000;130:954S-959S. [PubMed] |
| 25. | Vernon RG, Denis RG, Sørensen A. Signals of adiposity. Domest Anim Endocrinol. 2001;21:197-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Sahu A, Nguyen L, O'Doherty RM. Nutritional regulation of hypothalamic leptin receptor gene expression is defective in diet-induced obesity. J Neuroendocrinol. 2002;14:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333-339. [PubMed] |
| 28. | Pralong FP, Gaillard RC. Neuroendocrine effects of leptin. Pituitary. 2001;4:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Frühbeck G. Peripheral actions of leptin and its involvement in disease. Nutr Rev. 2002;60:S47-S55; discussion S68-S84, 85-87. [PubMed] |
| 30. | Heshka JT, Jones PJ. A role for dietary fat in leptin receptor, OB-Rb, function. Life Sci. 2001;69:987-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |