Published online Nov 15, 2003. doi: 10.3748/wjg.v9.i11.2587
Revised: December 6, 2002
Accepted: December 16, 2002
Published online: November 15, 2003
AIM: To report the long-term effect of stent placement in 115 patients with Budd-Chiari syndrome (BCS).
METHODS: One hundred and fifteen patients with BCS were treated by percutaneous stent placement. One hundred and two patients had IVC stent placement, 30 patients had HV stent placement, 17 of them underwent both IVC stent and HV stent. All the procedures were performed with guidance of ultrasound.
RESULTS: The successful rates in placing IVC stent and HV stent were 94% (96/102) and 87% (26/30), respectively. Ninety-seven patients with 112 stents (90 IVC stents, 22 HV stents) were followed up. 96.7% (87/90) IVC stents and 90.9% (20/22) HV stents remained patent during follow up periods (mean 49 mo, 45 mo, respectively). Five of 112 stents in the 97 patients developed occlusion. Absence of anticoagulants after the procedure and types of obstruction (segmental and occlusive) before the procedure were related to a higher incidence of stent occlusion.
CONCLUSION: Patients with BCS caused by short length obstruction can be treated by IVC stent placement, HV stent placement or both IVC and HV stent placement depending on the sites of obstruction. The long-term effect is satisfactory. Anticoagulants are strongly recommended after the procedure especially for BCS patients caused by segmental occlusion.
- Citation: Zhang CQ, Fu LN, Xu L, Zhang GQ, Jia T, Liu JY, Qin CY, Zhu JR. Long-term effect of stent placement in 115 patients with Budd-Chiari syndrome. World J Gastroenterol 2003; 9(11): 2587-2591
- URL: https://www.wjgnet.com/1007-9327/full/v9/i11/2587.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i11.2587
Budd-Chiari syndrome (BCS) is characterized by obstruction of outflow in hepatic vein (HV) and inferior vena cava (IVC) leading to hepatomegaly, portal hypertension, impaired liver function, formation of communicating channel, and edema in lower extremities. Various patterns of vascular obstruction can be seen in BCS. The most common type in the orient is short length obstruction (membranous and segmental) in IVC and/or in the ostium of main hepatic vein (HV), and most of them are chronic and idiopathic[1-3]; whereas thrombotic obstruction is the the most common cause in Western country[4,5].
The optimal management of BCS is difficulty, surgical shunting has been recommended as the most appropriate choice to relieve symptoms in most instances[6,7]. But the long-term patency of these shunts varied with high morbidity and mortality[7-9]. Orthotopic liver transplantation has been used to treat BCS cases, but it was mainly for patients with fulminant hepatic failure caused by acute BCS and those with end stage of cirrhosis[10,11]. Recently, the transjugular intrahepatic portosystemic shunt (TIPS) has been reported as an effective therapeutic method for BCS[12,13]. But primary TIPS shunt dysfunction occurred in 60% of patients with TIPS stent modifications, and angioplasties were required to keep a long-term patency[13,14].
With the development of percutaneous transluminal angioplasty (PTA) and stent placement in the 1990's, a pseudosurgical technique has been employed as an alternative to the major portosystemic shunts[15]. This procedure, applied by Furuil for the first time in a case of BCS in 1990[16], has shown beneficial results. Up to now, almost all of the reports were based on small numbers of patients without long-term follow-up. PTA and stent placement were limited to case report especially for patients with hepatic vein occlusion[17,18]. The role of these therapies in the overall management of BCS has not been clearly established.
From 1994, we have performed percutaneous IVC stent placement in 102 patients and HV stent placement in 30 patients with BCS, 17 out of 102 were treated with combined IVC and HV stent placement. Different from the other reports, all procedures were performed under ultrasound guidance instead of X-ray guidance. Our previous reports demonstrated the safety and advantages of IVC and/or HV stent placement under ultrasound guidance[19,20]. In this study, the long-term effects of stent placement in BCS were reported. The large series of patients enabled us to evaluate the outcome of stent placement and to establish protocol for management of BCS.
From April 1994 to June 2001, 115 patients with BCS underwent stent placement in our hospital (All were performed by Dr. Chunqing). There were 65 males and 50 females. The average age was 37.3 ± 12.7 years (SD, range 17-67). The duration of the illness ranged from 3 mo to 17 years. Underlying etiological factors for BCS were identified in 5 patients. Two patients had a history of tuberculosis infection, 2 patients took oral contraceptives, 1 patient was pregnant. No patients were examined for the levels of antithrombin-III, protein C and protein S. The main clinical features are listed in Table 1 according to the site of obstruction (see below). Patients manifested mainly as abdominal fullness, weakness, hepato-splenomegaly and ascites.
| Site of obstruction | |||
| IVC (n = 85) | HV (n = 13) | Combined (n = 17) | |
| Symptoms | |||
| Abdominal fullness | 79 | 13 | 17 |
| Weakness | 71 | 13 | 17 |
| Abdominal pain | 58 | 13 | 15 |
| Low extremities edema | 53 | 3 | 13 |
| Gastrointestinal bleeding | 9 | 4 | 8 |
| Jaundice | 5 | 4 | 7 |
| Hepatic encephalopathy | 3 | 2 | 3 |
| Signs | |||
| Hepatomegaly | 80 | 13 | 17 |
| Splenomegaly | 77 | 13 | 17 |
| Ascites | 36 | 10 | 12 |
| Distended abdominal veins | 55 | 3 | 5 |
| Leg ulcer | 21 | 0 | 3 |
| Management | IVC stent | HV stent | IVC and HV stent |
All patients underwent gray-scal sonography and colour Doppler sonography prior to the stent placement. Ultrasound scanning could identify the site, degree and extent of obstruction of hepatic IVC and hepatic veins, while colour Doppler could demonstrate the altered hemodynamic within the IVC and HV. In our early study only 11 patients underwent venography.
Based on the findings by ultrasound, colour Doppler and the subsequent probing of the lesions with a guide wire or a 5F-cathetor during the interventional procedure, the patients were divided into obstruction of inferior vena cava with at least one patent hepatic vein (IVC group, n = 85 patients), obstruction of three main hepatic veins (HV group, n = 13 patients), obstruction of both inferior vena cava and three main hepatic veins (Combined group, n = 17 patients). IVC stent were placed in both IVC group and combined group (102 patients). HV stent placement were performed in both HV group and combined group (30 patients). Of the102 patients who underwent IVC stent placement, 49 patients had membranous obstruction, and 53 patients had IVC segmental obstruction (range 1.0-7.6 cm, Table 2). While in 30 patients who underwent HV stent placement, 17 patients had membranous obstruction in HV and 13 patients had segmental obstruction in HV (range 1-4 cm, Table 2).
| Membraneous | Segmental (extent, cm) | |
| IVC Stenosis (n = 54) | 30 | 24 (1-7.6) |
| Occlusion (n = 48) | 19 | 29 (1-7.2) |
| HV Stenosis (n = 11) | 6 | 5 (1-4.5) |
| Occlusion (n = 19) | 11 | 8 (1-4.0) |
During the same period, sonography did not reveal hepatic IVC and the main hepatic vein in 30 patients, patients with thrombosis below the obstruction were excluded from this study.
All the procedures were performed under ultrasound guidance[19,20]. Before the procedure, all the patients were given cisapride 10 mg and pipemidic acid 0.5 three times daily for 3 d. They were fasted and asked to have bed rest for 12 h to reduce intestinal tympanites and to keep a clear image of ultrasound. In patients with massive ascites, before the procedure a therapeutic paracentesis was performed followed by intravenous administration of albumin. The methods were approved by the ethic committee of our hospital.
IVC stent placements: Briefly, the transducer of ultrasound unit was positioned in the infrasternal angle to show the longitudinal axis of hepatic IVC, the lesion and interventional devices. Though the right femoral vein, a 14F sheath was advanced over a guide wire into the IVC. Echo contrast in 5-10 mL normal saline was injected through the sheath. The soft end of a guide wire or a 5F-catheter was then introduced to probe the lesion under US guidance. If the obstruction was incompletely, the guide wire could easily cross the narrowed part of IVC into the right atrium. For complete obstruction, an 8-F Teflon catheter and its appropriate metal cannula were inserted through the sheath and were pushed carefully into the occluded IVC. If necessary, a Brockenbrough needle was inserted to cut through the lesion. Once the catheter or the needle was placed into right atrium, its strong rebound echo could be shown on US and blood return could be obtained. Then, a balloon catheter with a 1.8-2.4 cm diameter was inserted to dilate the IVC. At the end, under US guidance, the stent (Gianturco stent, Jayu Medical Equipment, Shenyang, China) was pushed into the obstructive part of IVC where it could completely support the obstructive portion after deployment.
HV stent placement: The procedures were performed by percutaneous and transhepatic route. Under ultrasound guidance, a 16-gauge needle was inserted into the hepatic vein via either an intracostal (for right HV) or infrasternal (for middle and left HV) approach. Then over a guide wire, a 10F sheath was put into HV, and all other operations were done thought the sheath to prevent damage of hepatic parenchyma. In patients with partially obstructed HV, the guide wire could cross the entrance of hepatic vein into IVC and finally into the right atrium. Otherwise, a Brockenbrough needle was used to make a tract from the hepatic vein into IVC. Then, the obstructive hepatic vein was dilated by a 1.0-1.2 cm diameter balloon catheter, and a metallic stent (Giantureco Z stent or Wallstent, Jayu Medical Equipment, Shenyang, China) was placed through the 10F catheter to support the hepatic vein. At the end, the 10-F sheath was withdrawn under guidance of ultrasound, and pieces of gelatin sponge were placed over the sheath to plug the tract.
After the procedure, antibiotics were given to all patients. Intravenous heparin was given for for 1 wk and followed by aspirin (75-100 mg per day) at least for 6 mo to prevent stent thrombosis.
During the follow-up period, manifestations of BCS were evaluated and the liver function was valued, and Doppler duplex ultrasonography was obtained to assess patency of the stent and the hemodynamics in HV and IVC. All patients underwent these examinations before discharge, and then they were seen at 3-6 mo intervals, or when they had recurrence of symptoms of BCS.
Results were expressed as mean ± SD, range or absolute numbers. χ² test, Wilcoxon's, or Student's t test was used. p < 0.05 was considered statistically significant.
IVC stent was placed successfully in 94% (95/102) of patients (IVC group and HV group). The procedure failed in 6 patients (all in IVC group) with segmental occluded IVC (3-4 cm). Pericardial effusion occurred in 3 patients and inferior infarction in 1 patient during dilatation of the occluded IVC with balloon, the Brockenbrough needle failed to cut through the hard occlusive segment of IVC in 2 patients. Stent migrated into right atrium in one patient who had mesoatrial shunt with the stent fixed to the wall of right atrium and no procedural death occurred.
Hemodynamic features in patients with successful stent placement improved significantly, the inferior vena cava pressure below the obstruction decreased from 29 ± 12.7 cm H2O to 11.5 ± 7.3 cm H2O (P < 0.05), and satisfactory antegrade flow in IVC was observed with a normal flow spectrum in colour Doppler ultrasound. All patients in IVC group improved clinically. Ascites, hepatomegaly, lower extremity edema, and distended abdominal veins disappeared or diminished at discharge.
Seventy-nine patients in IVC group were followed for 12-84 mo (mean 58 mo). Four patients were lost in follow-up. Of the remaining 75 patients, 48 had no symptoms, 24 improved clinically, but they still had mild weakness or abdominal swelling on physical examination. On the latest follow up, 68 patients were employed or engaged in full house keeping (Table 3). Of the 17 patients in combined group, 2 were lost to follow-up, the other 15 patients had no symptoms of IVC obstruction.
| IVC group | HV group | Combined group | |
| Number of patients | 75 | 7 | 15 |
| Follow-up (months) | |||
| Mean | 49 | 45 | 45 |
| Range | 7-84 | 9-78 | 9-78 |
| Ascites | |||
| Before procedure | 29 | 7 | 11 |
| Disappeared | 21 | 5 | 8 |
| improved | 7 | 2 | 3 |
| Hepatomegaly | |||
| Before procedure | 73 | 7 | 15 |
| Disappeared | 38 | 3 | 6 |
| Improved | 33 | 3 | 7 |
| Splenomegaly | |||
| Before procedure | 67 | 7 | 15 |
| Disappeared | 9 | 1 | 3 |
| Improved | 46 | 3 | 8 |
| Abnormal liver function test | |||
| Before procedure | 53 | 7 | 15 |
| Disappeared | 21 | 3 | 4 |
| Improved | 29 | 3 | 9 |
| Employed or housekeeping | 68 | 6 | 14 |
| Stent occlusion | 3 | 1 | 1 |
Thirty-four of the 90 patients (75 in IVC group and 15 in combined group) were followed up for 5 years, 31 patients for 3 years, 25 patients for 1 year. Doppler ultrasonography showed that IVC stent was patent and worked effectively in 87 patients. The overall IVC stent patency rate was 96.6% (87/90). Stent occlusion occurred in 3 patients at 12-24 mo (mean 19 mo) following stent placement, these 3 patients had recurrence of abdominal fullness and edema of lower extremity, and 1 patient had ascites. Two patients underwent caval-portal shunt and the others were treated with diuretics and anticoagulants.
Hepatic vein stent was placed in 30 patients, including 13 patients in HV group and 17 in combined group. In HV group, the patients had hepatic vein stent placement alone, although some patients had narrowed IVC pressed by enlarged caudate lobe. In combined group, successful IVC stent placement resulted in disappearance of all symptoms of IVC obstruction. However, ascites, and hepatosplenomegaly were not alleviated. Therefore, hepatic vein stent was placed 1 wk after IVC stent placement.
HV stent were successfully placed in 86.6% (26/30) patients. Four patients in HV group failed due to a long occluded hepatic vein (3.0-3.5 cm), which was difficult to cut through. All but one of the 26 successful patients had hemodynamic improvement immediately after the procedure. HV pressure dropped from 36.5 ± 16.4 cm H2O to 12.7 ± 9.5 cm H2O and satisfactory antegrade flow was noted with a normal phasic flow spectrum in the stented HV. At the time of discharge, 10 patients were free of ascites, the massive ascites decreased without diuretics, and hepato-splenomegaly disappeared or diminished obviously in 25 patients.
Early stent occlusion occurred in one patient of HV group on the third day after the procedure, because the stent immigrated into the hepatic vein and the ostium of hepatic vein was not support by the stent. The patient was treated with repeated paracentesis, intravenous albumin, and a portocaval shunt. Severe haemorrhage from the unplugged transhepatic access occurred in one patient in our early study. Emergent surgical haemostasis was performed, and the patient survived well with a patent hepatic vein stent. One patient with peritonitis and ascitis was treated with paracentesis and parenteral antibiotics. Two patients experienced pleural effusion resolved within 2 wk. No other major complications occurred.
During a mean follow-up period of 53 mo (range 15-78 mo), three patients missed the follow-up (1 in HV group, 2 in combined group). Of the 22 follow-up patients (7 in HV group, 15 in combined group), 7 patients were followed up for 5 years, 11 for 3 years, 4 for 1 year. Clinical symptoms and signs of BCS patients improved (Table 3). In these patients, ascites disappeared or decreased, hepatomegaly and splenomegaly were greatly alleviated or dissapeared. Periodic liver function tests were consistent normal or improved. Colour Doppler ultrasonography examinations demonstrated patency of the stents in hepatic vein in 20 patients. The overall HV stent patency rate was 90.9% (20/22). All were gainfully employed and leading productive lives of good quality. One woman married, pregnant and delivered a healthy baby.
Ascites recurred in 1 patient in combined group at 6 mo after the procedure, varices bleeding occurred in 1 patient in HV group at 12 mo after the stent placement. Doppler ultrasonography confirmed the stent occlusion and dysfunction in 2 patients. One patient underwent mesocaval shunt, the other was treated with diuretics.
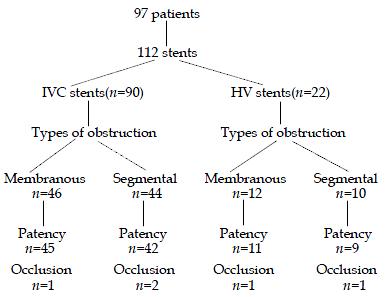
Of the 112 stents in 97 follow-up patients, occlusion was observed in 5 (4.5%) stents (3 in IVC stents, 2 in HV stents) (Figure 1). Risks of stent occlusion were evaluated (Table 4), and stent occlusion in HV stents (9.1%) was more common than that in IVC stents (3.3%) (P < 0.05). Absence of long-term anticoagulants was related to stent occlusion, stent occlusion was observed in 3 out of 25 (12%) stents in patients without anticoagulant therapy versus 2 of 87 (2.3%) stents in patients with at least 6 mo of anticoagulants for.
| Number of stents | Number of occlusion | P valve | |
| Total | 112 | 5 | |
| Site of stents | |||
| IVC | 90 | 3 | |
| HV | 22 | 2 | < 0.05 |
| Degrees of obstruction | |||
| Stenosis | 60 | 2 | |
| Occlusion | 52 | 3 | > 0.05 |
| Types of obstructon | |||
| Membraneous | 58 | 2 | |
| Segmental | 54 | 3 | > 0.05 |
| Anticoagulants at least 6 mo | |||
| Yes | 87 | 2 | |
| No | 25 | 3 | < 0.05 |
No significant difference was found between stent occlusion and severity of obstruction (Table 4). Stent occlusion occurred in 11% (3/27) patients with segmental obstruction and occlusion versus 2.9% (1/34) patients without them, the difference was significant (P < 0.05).
Since the location, extent and rapidity of venous obstruction are highly variable, a range of clinical presentations necessitates an individualized therapeutic strategy. The management of BCS has traditionally been classified as medical, surgical and radical. Conventional medical therapy with diuretics and anticoagulation has been reported to be of limited value in relieving hepatic venous outflow obstruction[21]. The use of fibrinolytic therapy might be of benefit for patients at early stage of acute thrombosis[23]. Surgical treatment was the most frequently reported approach for BCS[6,7]. When venous obstruction is limited to the main HV without serious involvement of IVC, a portacaval shunt or a mesocaval shunt is proposed. In cases of BCS complicated with obstruction of IVC, the mesoatrial shunt may be used to allow the portal flow to drain directly into the right atrium. Liver transplantation as the second surgical option for BCS was indicated for BCS in acute or chronic liver failure in the Western world[23,24]. Radiological interventions including balloon angioplasty, stent insertion and transjugular intrahepatic portosystemic shunts have been shown to be effective for selected patients with BCS during the past 10 years[11,25], but these results were confined to small series of patients with short term follow up.
In this series, all BCS patients were caused by short length obstruction of the hepatic IVC and/or of the main HV. Most patients were chronic and idiopathic. Our series was the largest report to date for the use of stent placement in BCS. The results demonstrated the long-term efficacy of stent placement for BCS.
In this series, stent placement was performed based on the locations of vascular obstruction.
IVC obstruction with short length lesion was a common cause for BCS in the Eastern countries including China[2,3,26]. Although balloon angioplasty has been regarded as the first choice for patients with IVC obstruction, restenosis and redilitation was reported[2,27], even in patients with membranous obstruction[28]. In our study, the residual membrane in most cases was often pushed back into the lumen of IVC once the balloon catheter moved back. This might be a cause of reccurrence of BCS. Therefore, stents were placed in the restricted part in all patients whether the lesion was a membraneous or a segmental obstruction. During a period of 12-84 mo follow-up, 87 out of 90 (96.7%) IVC stents remained patent and functioned well, the manifestions of BCS disappeared or improved in all the patients. The results demonstrate that IVC stent placement is effective and reliable in management of BCS and has a satisfactory long-term patency.
HV obstructon was one of the main causes of BCS, both in Eastern and in Western countries[2,29]. However, treatment of HV obstruction has been challenging. Usually the hepatic vein angioplasty or stent were performed under X-ray guidance via the jugular vein or both transhepatic and jugular veins. Under this condition, the ostium of hepatic vein could not be revealed directly. Thus, it was very difficult to cut through the occluded orifice of the hepatic vein from the vena cava, cardiac perforation or IVC rupture was reported during the procedure[30,31]. Up to now, reports on treatment on HV obstruction were limited with a small number of patients, and its successful rate was low[2,25]. We performed percutanous hepatic vein recanalization, dilation and stent placement in 26 out of 30 patients with a success rate of 86.7%. This was a report involving the treatment of BCS with short length hepatic vein obstruction. All the patients had three obstructed hepatic veins, we chose to place stent in one of them. By intrahepatic communicating channels of hepatic veins, the stent was sufficient to drain the entire liver. During a period of 15-78 mo follow-up, 90.9% (20/22) patients had patent stents, the clinical manifestations of BCS improved obviously or disappeared. The long term stent patency was rather good compared with PTA of hepatic vein that had a high incidence of restenosis[31]. In our study, percutanous trashepatic hepatic vein stent placement was the first choice in the treatment of BCS caused by short length hepatic vein obstruction.
For the treatment of HV obstruction, percutanous transhepatic route was safe if the tract was plugged with gelatin sponge before removal of the sheath. Patients with short length HV obstruction should be treated by stent placement instead of simple angioplasty, because hepatic vein webs were more likely to restenose compared with caval webs following angioplasty[31]. Performance under ultrasound guidance contributed to a high success rate and low morbidity, reducing intestinal tympanites and ascites before the procedure was necessary for a clean image of ultrasound.
Combined IVC and HV obstruction was a special kind of BCS consisting of 15% of our patients. Management of these patients was usually difficult[2,6,7]. When the procedures were divided into 2 steps, the treatment became simple. In 17 patients, the IVC stent were placed first, and the HV stent was placed one week later. During a long-term follow-up, only one patient developed HV stent occlusion, and all IVC stents worked well. The results showed that the combined procedure was an advisable choice for BCS patients with both IVC and HV obstruction.
The total stent occlusion rate was 4.5% during the follow-up. The long term results were satisfactory. The stent occlusion was more likely to occurred in BCS patients with segmental and occluded obstruction than in patients with membraneous and stenosis obstruction, the stent occlusion rate was higher in patients without anticoagulants after operation than that in patients with anticoagulants at least for 6 mo. Stent occlusion occurred more likely at HV (9.1%) than at IVC (3.3%). Therefore, we suggest that anticoagulants should be used routinely after the procedure, especially for patients with segmental and occluded obstruction, and for patients having HV stent placement.
In conclusion, IVC and HV stents or combined IVC and HV stent can be applied to BCS patients with short length obstruction depending on the sites of obstruction. The stents can be placed under ultrasound guidance with a high successful rate and a low morbidity. Excellent long-term results can be obtained in IVC and HV stents as well as in combined stents. Anticoagulants are strongly recommended for at least 6 mo after the procedure.
Edited by Ren SL and Wang XL
| 1. | Okuda H, Yamagata H, Obata H, Iwata H, Sasaki R, Imai F, Okudaira M, Ohbu M, Okuda K. Epidemiological and clinical features of Budd-Chiari syndrome in Japan. J Hepatol. 1995;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 144] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Kohli V, Pande GK, Dev V, Reddy KS, Kaul U, Nundy S. Management of hepatic venous outflow obstruction. Lancet. 1993;342:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Dilawari JB, Bambery P, Chawla Y, Kaur U, Bhusnurmath SR, Malhotra HS, Sood GK, Mitra SK, Khanna SK, Walia BS. Hepatic outflow obstruction (Budd-Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (. Baltimore). 1994;73:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 215] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Mahmoud AE, Mendoza A, Meshikhes AN, Olliff S, West R, Neuberger J, Buckels J, Wilde J, Elias E. Clinical spectrum, investigations and treatment of Budd-Chiari syndrome. QJM. 1996;89:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Mitchell MC, Boitnott JK, Kaufman S, Cameron JL, Maddrey WC. Budd-Chiari syndrome: etiology, diagnosis and management. Medicine (. Baltimore). 1982;61:199-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 205] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Slakey DP, Klein AS, Venbrux AC, Cameron JL. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Orloff MJ, Daily PO, Orloff SL, Girard B, Orloff MS. A 27-year experience with surgical treatment of Budd-Chiari syndrome. Ann Surg. 2000;232:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Panis Y, Belghiti J, Valla D, Benhamou JP, Fékété F. Portosystemic shunt in Budd-Chiari syndrome: long-term survival and factors affecting shunt patency in 25 patients in Western countries. Surgery. 1994;115:276-281. [PubMed] |
| 9. | Bismuth H, Sherlock DJ. Portasystemic shunting versus liver transplantation for the Budd-Chiari syndrome. Ann Surg. 1991;214:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Klein AS, Sitzmann JV, Coleman J, Herlong FH, Cameron JL. Current management of the Budd-Chiari syndrome. Ann Surg. 1990;212:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Tilanus HW. Budd-Chiari syndrome. Br J Surg. 1995;82:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Ganger DR, Klapman JB, McDonald V, Matalon TA, Kaur S, Rosenblate H, Kane R, Saker M, Jensen DM. Transjugular intrahepatic portosystemic shunt (TIPS) for Budd-Chiari syndrome or portal vein thrombosis: review of indications and problems. Am J Gastroenterol. 1999;94:603-608. [PubMed] |
| 13. | Perelló A, García-Pagán JC, Gilabert R, Suárez Y, Moitinho E, Cervantes F, Reverter JC, Escorsell A, Bosch J, Rodés J. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medical therapy. Hepatology. 2002;35:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Cejna M, Peck-Radosavljevic M, Schoder M, Thurnher S, Ba-Ssalamah A, Angermayr B, Kaserer K, Pokrajac B, Lammer J. Repeat interventions for maintenance of transjugular intrahepatic portosystemic shunt function in patients with Budd-Chiari syndrome. J Vasc Interv Radiol. 2002;13:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Carrasco CH, Charnsangavej C, Wright KC, Wallace S, Gianturco C. Use of the Gianturco self-expanding stent in stenoses of the superior and inferior venae cavae. J Vasc Interv Radiol. 1992;3:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Furui S, Sawada S, Irie T, Makita K, Yamauchi T, Kusano S, Ibukuro K, Nakamura H, Takenaka E. Hepatic inferior vena cava obstruction: treatment of two types with Gianturco expandable metallic stents. Radiology. 1990;176:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Weernink EE, Huisman AB, ten Napel CH. Treatment of Budd-Chiari syndrome by insertion of wall-stent in hepatic vein. Lancet. 1991;338:644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Dolmatch BL, Cooper BS, Chang PP, Gray RJ, Horton KM. Percutaneous hepatic venous reanastomosis in a patient with Budd-Chiari syndrome. Cardiovasc Intervent Radiol. 1995;18:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Zhang C, Fu L, Zhang G, Xu L, Shun H, Wang Z, Zhu J. Ultrasonically guided inferior vena cava stent placement: experience in 83 cases. J Vasc Interv Radiol. 1999;10:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Chunqing Z, Lina F, Guoquan Z, Lin X, Zhaohai W, Tao J, Chengyong Q, Juzhen Z. Ultrasonically guided percutaneous transhepatic hepatic vein stent placement for Budd-Chiari syndrome. J Vasc Interv Radiol. 1999;10:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | McCarthy PM, van Heerden JA, Adson MA, Schafer LW, Wiesner RH. The Budd-Chiari syndrome. Medical and surgical management of 30 patients. Arch Surg. 1985;120:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Mitchell MC, Boitnott JK, Kaufman S, Cameron JL, Maddrey WC. Budd-Chiari syndrome: etiology, diagnosis and management. Medicine (. Baltimore). 1982;61:199-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 205] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Hemming AW, Langer B, Greig P, Taylor BR, Adams R, Heathcote EJ. Treatment of Budd-Chiari syndrome with portosystemic shunt or liver transplantation. Am J Surg. 1996;171:176-180; discussion 180-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Rao AR, Chui AK, Gurkhan A, Shi LW, Al-Harbi I, Waugh R, Verran DJ, McCaughan GW, Koorey D, Sheil AG. Orthotopic liver transplantation for treatment of patients with Budd-Chiari syndrome: A Singe-center experience. Transplant Proc. 2000;32:2206-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Pisani-Ceretti A, Intra M, Prestipino F, Ballarini C, Cordovana A, Santambrogio R, Spina GP. Surgical and radiologic treatment of primary Budd-Chiari syndrome. World J Surg. 1998;22:48-53; discussion 53-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Singh V, Sinha SK, Nain CK, Bambery P, Kaur U, Verma S, Chawla YK, Singh K. Budd-Chiari syndrome: our experience of 71 patients. J Gastroenterol Hepatol. 2000;15:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | De BK, Biswas PK, Sen S, Das D, De KK, Das U, Mandal SK, Majumdar D. Management of the Budd-Chiari syndrome by balloon cavoplasty. Indian J Gastroenterol. 2001;20:151-154. [PubMed] |
| 28. | Hung WC, Fang CY, Wu CJ, Lo PH, Hung JS. Successful metallic stent placement for recurrent stenosis after balloon angioplasty of membranous obstruction of inferior vena cava. Jpn Heart J. 2001;42:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Valla D, Hadengue A, el Younsi M, Azar N, Zeitoun G, Boudet MJ, Molas G, Belghiti J, Erlinger S, Hay JM. Hepatic venous outflow block caused by short-length hepatic vein stenoses. Hepatology. 1997;25:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Lois JF, Hartzman S, McGlade CT, Gomes AS, Grant EC, Berquist W, Perrella RR, Busuttil RW. Budd-Chiari syndrome: treatment with percutaneous transhepatic recanalization and dilation. Radiology. 1989;170:791-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Fisher NC, McCafferty I, Dolapci M, Wali M, Buckels JA, Olliff SP, Elias E. Managing Budd-Chiari syndrome: A retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut. 1999;44:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |