Published online Nov 15, 2003. doi: 10.3748/wjg.v9.i11.2539
Revised: June 1, 2003
Accepted: June 7, 2003
Published online: November 15, 2003
AIM: The present study was undertaken to purify and partially characterize the 33.5-kilodalton (33.5 kDa) vesicular protein in human bile and to explore the possible molecular mechanisms of the initial crystal nucleation process.
METHODS: The 33.5 kDa vesicular protein was isolated by ultracentrifugation and further purified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions. The purified 33.5 kDa vesicular protein was subjected to N-terminal amino acid sequencing and amino acid analysis. Cholesterol crystallization activity was detected by cholesterol crystal growth assay. The sugar chain of the 33.5 kDa vesicular protein was analyzed by dot-immunobinding assay of lectin coupled to a peroxidase (HRP-DSA, HRP-ConA, HRP-WGA) and was deglycosylated using two different enzymatic approaches (N-deglycosylation and O-deglycosylation) to determine the molecular weight of the protein component, the type of linkage between polypeptide and carbohydrate components.
RESULTS: The 33.5 kDa vesicular protein with complicated glycan was an extensively glycosylated (37.3%) monomer and these sugar chains strongly bound to DSA, but did not bind to ConA. Amino acid sequencing indicated that the protein was unique. The 33.5 kDa vesicular protein exhibited potent cholesterol crystallization promoting activity in vitro with derived crystal growth curve indices It, Ig, Ic presented as 0.57, 1.52, and 1.63 respectively. Both enzymatic proteolysis and N-deglycosylation of the protein removed all activity.
CONCLUSION: These data suggest the 33.5 kDa vesicular protein may be responsible for the pathogenesis of cholesterol gallstone disease, and the sugar chains play an important role in pro-nucleating process.
- Citation: Xiang JB, Cai D, Ma BJ, Cha XL, Wang LY, Mo HQ, Zhang YL. Purification and characterization of 33.5 kDa vesicular protein in human bile. World J Gastroenterol 2003; 9(11): 2539-2543
- URL: https://www.wjgnet.com/1007-9327/full/v9/i11/2539.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i11.2539
Cholesterol nucleation process represents a critical step in the cholesterol gallstone formation. Cholesterol pro-nucleating and anti-nucleating proteins can accelerate or retard the rate of cholesterol crystallization in supersaturated bile, and thus may play important roles in cholesterol crystallization[1-3]. From 1988, both inhibitors and promoters of cholesterol crystallization have been isolated from human bile and characterized[4-7]. The major cholesterol crystallization promoting activity was localized at the concanavalin A-binding fraction of biliary glycoproteins (CABG). These proteins include mucin[8], immunoglobulins[9-11], α1-acid protein[12], aminopeptidase N[4], low-density protein-lipid complex[5,13], and some unidentified proteins such as 70 kDa[14] and 200 kDa[15] pro-nucleating glycoproteins. Abei et al[16] provided comparative data regarding the relative potency of these different glycoprotein promoters and found that α1-acid protein accounted for the greatest portion (33%) of the net biliary Con A-bound promoting activity derived from currently defined and well-identified glycoproteins. But still more than 60% of total Con A-bound promoting activity remains unaccounted for. It was speculated that there was still some other more important proteins involved in cholesterol nucleation process.
Lecithin vesicles are the primary cholesterol carriers in bile supersaturated with cholesterol and have been shown to play an important role in the nucleation of cholesterol. This nucleation takes place after aggregation and fusion of cholesterol-rich biliary vesicles, a process modulated by biliary proteins. Miquel et al[17] found a potent cholesterol pro-nucleating activity in purified biliary vesicles. Further study demonstrated that this activity was related with specific vesicular proteins including immunoglobulins IgA, IgG and IgM[18].
In this study, a novel 33.5 kDa vesicular protein obtained from human gallbladder bile of cholesterol gallstone patients was isolated, purified and partially characterized. We attempted to determine whether pro-nucleating activity occurred in the 33.5 kDa vesicular protein and to detect whether the protein was lectin-specific. Our results showed that the 33.5 kDa vesicular protein exhibited potent pro-nucleating activity in vitro, which depends on intact structure of peptide and sugar chain, and especially bound DSA lectin.
Sodium salts of taurocholic (STC) and taurodeoxycholic (STDC) greater than 99% purity, cholesterol (CH), egg lecithin and Tween 20 were obtained from Fluka Company. Metrizamide, nitrocellulose sheets, and all the chemicals for SDS-PAGE were obtained from Sigma Chemical Co. Datura stramonium agglutinin (DSA), wheat germ agglutinin (WGA), concanavalin A (Con A), peroxidase (HRP), and Sephadex G150 were also from Sigma Chemical Co. Periodic acid (NaIO4) was purchased from Wako Pure Chemical. N-glycosidase F, endo-α-N-acetyl-galactosaminidase, neuraminidase, and Pronase K were purchased from Boehringer Mannheim Corp., Germany, and 0.22 μm micropore filters were obtained from Millipore Corp., Bedford, MA. USA.
Patients and bile collection All patients gave written informed consent to participate in the study, which was approved by the ethical committee. Gallbladder bile was obtained from three patients by directly puncturing the gallbladder with a sterile 19G needle at cholecystectomy for cholelithiasis. The bile (20 mL) was immediately transported to the laboratory and stored at -80 °C until processed.
Protein purification procedure Pooled bile specimens were separated on a molecular sieving chromatography column (BioGel A-5m, 5 × 100 cm), eluted with 10 mmol/L Tris-HCl buffer to remove soluble mucin glycoprotein. The main fraction was centrifuged at 10000 rev/min for 10 min at room temperature. The upper fraction was filtered through 0.22 μm micropore filters, and metrizamide (13% w/v) was directly dissolved in the elution and centrifuged at 45000 rev/min for 3.5 h at 10 °C in a Vti-50 vertical rotor (Beckman Instruments Inc., USA). The top opalescent vesicular fraction was collected by tube puncturing and loaded on SDS-PAGE under nonreducing conditions. The 33.5 kDa vesicular protein lane was resected according to the protein marker position and dialyzed in Tris-HCl buffer and concentrated as Ma et al[19] described.
SDS-PAGE SDS-PAGE (5%-12%) was developed in a buffer system described by Laemmli[20]. Aliquots (100 μL) of protein and bile samples were resolubilized with a sample buffer (60 mmol/L Tris-HCl, 2% SDS, 10% glycerol, pH6.8). On completion of the electrophoretic run, gels were fixed in a 50% methanol, 10% acidic acid solution for 6 h and stained with Coomassie blue.
Preparation of lectin-HRP conjugate The lectin-HRP conjugate of DSA-HRP, WGA-HRP and Con A-HRP was made according to Guo et al[21]. Briefly, 5 mg HRP was dissolved in 0.5 mL distilled water, then added with 0.5 mL 60 mmol/L NaIO4 and kept at 4 °C for 30 min. Five mg lectin such as DSA, WGA and Con A was mixed with HRP and 0.1 mol/L α-methyl mannose for Con A, and N-acetylglucosamine for DSA and WGA was added to protect the glycan binding site of the lectin. The reaction mixture was dialyzed in 50 mmol/L carbonate buffer (pH9.5) and centrifuged at 4000 rev/min for 10 min. The supernatant was removed and the pellet was dissolved and dialyzed in sodium phosphate buffer (20 mmol/L, pH7.4).
Lectin affinity staining Five, 10, 15 μg/mL of purified 33.5 kDa vesicular proteins were blotted to nitrocellulose membrane respectively. The membrane was blocked with 1% BSA overnight at 37 °C. Subsequent incubation of the membrane with 1:500 peroxidase-labeled Datura stramonium agglutinin (DSA), wheat germ agglutinin (WGA), concanavalin A (Con A) in the same solution was followed by washing three times in the TTBS buffer (0.05% Tween 20, 0.1 mol/L Tris-HCl, pH7.5) and chemiluminescent detection.
Amino acid analysis The purified 33.5 kDa vesicular protein was hydrolyzed for 16 h at 115 °C in 6 N HCl/0.2% phenol containing norleucine as an internal standard. After incubation, samples were dried and redissolved in 100 μL of NaS sample dilution buffer (Beckman Instruments Inc., USA) and run on a Beckman model 7300 Amino Acid Analyzer.
Amino acid sequencing The amino-terminal sequences of the 33.5 kDa vesicular protein were subjected to N-terminal amino acid sequencing with an automated sequencer (model 477A: Protein Sequencer, Applied Biosystems). Determined sequences were compared with those well-identified glycoproteins in the Pub-Med NCBI human gene bank database.
Enzymatic deglycosylation The 33.5 kDa vesicular protein was treated with N-glycanase enzyme according to supplier’s specifications based on the work of Elder and Plummer et al[22,23]. Five hundred μg 33.5 kDa vesicular protein boiled for 5 min was diluted with 0.1 mmol/L sodium phosphate buffer, pH8.6, 10 mmol/L 1,10-phenanthroline, and then mixed with 10 U N-glycanase, and the reaction mixture was incubated for 24 h at 37 °C. The molecular weight of deglycosylated polypeptide backbone was then detected using SDS-PADE.
In the O-deglycosylation study, the vesicular protein was diluted with 10 mmol/L calcium acetate, 20 mmol/L sodium cacodylate buffer (pH7.0) and was incubated with 10 U/mL of neuraminidase for 12 h at 37 °C. This was followed by further incubation with 2 U/mL of endo-α-N-acetyl-galactosaminidase for 12 h at 37 °C. Finally, the mixture was examined using SDS-PAGE.
Proteolysis studies One hundred μg of 33.5 kDa vesicular protein was dissolved in 50 μL ammonium bicarbonate (25 mmol/L, pH11), and then incubated with 1.5 U Pronase K for 24 h at 37 °C. After incubation, the sample was concentrated and loaded on SDS-PAGE.
Cholesterol crystal growth assay Supersaturated model bile was prepared with a cholesterol saturation index of 1.4, a total lipid concentration of 125 g/L, and a bile acid/phospholipid ratio of 4.4. This model bile was made as Busch et al[24,25] described. In brief, this lipid mixture was evaporated to dryness, lyophilized, and then resolubilized with 20 mmol/L Tris-HCl/150 mmol/L NaCl (TBS), pH7.4 at 55 °C. After filtration (0.22 μm), 25 μL of this model bile mixed with 50 μg protein or its enzymatic samples was diluted with 475 μL TBS/10 mmol/L STDC solution. After 20 min, absorbance at a single wavelength within the visible range (700 nm) was sequentially measured. The cholesterol crystal growth curves of the supersaturated model bile without (control) and with (experimental) protein samples were thus generated for each sample. The three growth curve parameters were derived: growth index Ig = maximal slope of experimental curve/maximal slope of control, crystal index Ic = final crystal concentration of experimental/final crystal concentration of control, time index It = onset time of experimental/onset time of control.
The cholesterol crystal growth curves were compared by using analysis of variance (ANOVA) at each time to determine whether difference existed between the study groups. When the ANOVA was statistically significant (P < 0.05), the Dunnett’s multiple comparison procedure was made to compare each of the study groups to the control group.
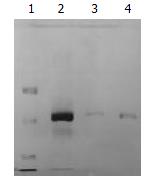
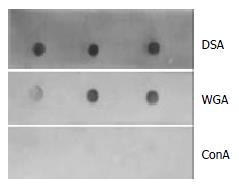
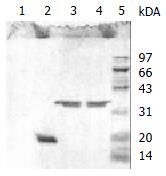
The bile was divided into three fractions after ultracentrifugation (Figure 1). The top opalescent vesicular fraction was collected by tube puncture and the targeted vesicular protein was further separated by SDS-PAGE. The protein profile from three different gallstone patients with Coomassie blue staining is shown in Figure 2. The protein marker is shown at lane 1 and a single band of 33.5 kDa protein at lanes 2-4 on SDS-PAGE was stained under nonreducing condition. Amino acid analysis of the purified glycoprotein showed that the protein was composed of 153 amino acid residues of which almost one third were the following amino acids: glutamine/glutamic acid and asparagines/aspartic acid (Table 1). N-amino-terminal sequencing of the protein showed H2N-Asp-Asn-Ser-Gln-His-Arg-Tyr-Val-Phe-Ile, which was different from α1-acid protein, Ig, aminopeptidase N and phospholipase C. Lectin staining showed higher affinity for Datura stramonium agglutinin (DSA) than for wheat germ agglutinin (WGA) and concanavalin A (Con A) (Figure 3). N-deglycosylation studies showed disappearance of the original 33.5 kDa protein and the presence of a new 21 kDa band on SDS-PAGE (Figure 4), indicating the protein was heavily glycosylated (37.3%) and the connection mode between polypeptide and carbohydrate components was N-linkage. Proteolysis studies showed the protein was sensitive to Pronase K digestion.
| Amino acid | nmol/total protein | No. of residues/mol protein |
| Asp/Asn | 6.761 | 19 |
| Thr | 4.488 | 13 |
| Ser | 1.589 | 5 |
| Glu/Gln | 10.434 | 30 |
| Gly | 2.242 | 6 |
| Ala | 2.864 | 8 |
| Val | 2.501 | 7 |
| Ile | 3.226 | 9 |
| Leu | 4.782 | 14 |
| Tyr | 1.937 | 6 |
| Phe | 2.966 | 8 |
| Lys | 4.777 | 14 |
| His | 0.840 | 2 |
| Arg | 2.645 | 8 |
| Pro | 1.411 | 4 |
| NH2 | 11.297 | 32 |
| Total | 64.76 | 153 |
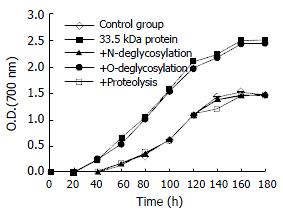
Figure 5 depicts the promoting effect of 33.5 kDa vesicular protein on cholesterol crystal growth curve at the concentration of 100 μg/mL. The protein strongly promoted cholesterol crystallization, accelerated the onset and increased the total quantity of crystal plates with derived crystal growth curve indices It, Ig, Ic presented as 0.57, 1.52, 1.63 respectively. But no promoting activity was detected in the same supersaturated model bile after incubation with N-glycanase enzyme or complete protein degradation (Table 2).
| It | Ig | Ic | P value | |
| Purified 33.5 kDa protein | 0.57 | 1.52 | 1.63 | < 0.05a |
| + N-deglycosylation | 1.08 | 1.01 | 0.98 | < 0.05b |
| + O-deglycosylation | 0.58 | 1.61 | 1.54 | < 0.05a |
| + Proteolysis | 1.12 | 0.87 | 0.99 | < 0.05b |
Since the first report of the presence of pro-nucleating activity in cholesterol patient’s bile by Burnstein et al[26], many groups have tried to purify and identify the active protein-related components[16,17,25,27,28]. Of particular interest are the presence and role of concanavalin A-binding fraction of biliary glycoproteins (CABG), which have a potent cholesterol crystallization-promoting activity. Proteins thought to explain this activity included α1-acid protein[12], immunoglobulins[9-11], aminopeptidase N[4,6], and a pronase resistant carcinoembryonic antigen-related cell adhesion molecule 1 most recently described by Jirsa et al[29], and some unidentified proteins such as 200 kDa pro-nucleating glycoprotein[15]. But still most of the activity has not been identified[30]. In this study we purified and characterized a novel promoting-nucleation glycoprotein with molecular weight of 33.5 kDa in vesicular bile of cholesterol gallstone patients. In 1992, Miquel et al[17] isolated and purified human vesicles with potent cholesterol-nucleation-promoting activity, and found that this protein-related activity belonged to immunoglobulins. Although they were from the same vesicular bile, the difference between the immunoglobulin family of glycoprotein and the 33.5 kDa vesicular protein was obvious. We took considerable care to rule out the possibility that the present glycoprotein shared similar features with the immunoglobulins. First, the potent cholesterol-nucleation-promoting vesicular protein had a strong activity of accelerating the onset and increasing the total quantity of crystals appearance and was unique to have a high affinity for Datura stramonium agglutinin (DSA), and did not bind to concanavalin A (Con A). This was different from the previously described promoting-nucleation glycoprotein. Amino acid sequencing study further demonstrated that the 33.5 kDa vesicular protein with N-amino-terminal sequencing of H2N-Asp-Asn-Ser-Gln-His-Arg-Tyr-Val-Phe-Ile, was a novel glycoprotein from vesicular bile.
In additional experiments, the 33.5 kDa vesicular protein could not only accelerate onset of nucleation, but also induce rapid cholesterol crystallization growth. We speculate the factor identified in this study may play an important role in the initial stage of the gallstone formation. To study the underlying mechanism and pathophysiological significance of the peptide and carbohydrate moiety, the 33.5 kDa vesicular protein was treated with glycanase enzyme and pronase respectively. Incubation with N-glycanase resulted in disappearance of the original 33.5-kilodalton band and presence of a strong 21-kilodalton band on SDS-PAGE, and no cholesterol crystallization promoting activity of 33.5 kDa vesicular protein was detected in supersaturated model bile. It suggested that the sugar chain might be responsible for the promoting-nucleation activity. This striking characteristic of the vesicular protein was very similar to α1-acid protein. Abei et al[12] reported that α1-acid protein was 37% glycosylated with mannose, sialic acid content, and some other multiple antennae and the carbohydrate moiety were essential to the promoting activity of glycoprotein. In addition, vesicular glycoprotein was completely degradated and no promoting activity existed after proteolytic digestion.
In conclusion, our results indicate that, the 33.5 kDa vesicular protein with complicated glycan and high affinity for DSA, is a novel and unique pro-nucleating glycoprotein, which exhibits potent cholesterol crystallization promoting activity in vitro. However, further studies are needed to evaluate the predictive value, concentration, relative potency and origin of the 33.5 kDa vesicular protein before we can ascertain its specific role in the pathogenesis of cholesterol gallstone disease.
The skillful technical assistance of Dr. Chuan Xin Huang and Dr. Jia Da Li is gratefully acknowledged.
Edited by Zhu LH and Wang XL
| 1. | Miquel JF, Van Der Putten J, Pimentel F, Mok KS, Groen AK. Increased activity in the biliary Con A-binding fraction accounts for the difference in crystallization behavior in bile from Chilean gallstone patients compared with Dutch gallstone patients. Hepatology. 2001;33:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Nunes DP, Afdhal NH, Offner GD. A recombinant bovine gallbladder mucin polypeptide binds biliary lipids and accelerates cholesterol crystal appearance time. Gastroenterology. 1999;116:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Luk AS, Kaler EW, Lee SP. Protein lipid interaction in bile: effects of biliary proteins on the stability of cholesterol-lecithin vesicles. Biochim Biophys Acta. 1998;1390:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Groen AK, Noordam C, Drapers JA, Egbers P, Jansen PL, Tytgat GN. Isolation of a potent cholesterol nucleation-promoting activity from human gallbladder bile: role in the pathogenesis of gallstone disease. Hepatology. 1990;11:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Zijlstra AI, Offner GD, Afdhal NH, van Overveld M, Tytgat GN, Groen AK. The pronase resistance of cholesterol-nucleating glycoproteins in human bile. Gastroenterology. 1996;110:1926-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Núñez L, Amigo L, Mingrone G, Rigotti A, Puglielli L, Raddatz A, Pimentel F, Greco AV, González S, Garrido J. Biliary aminopeptidase-N and the cholesterol crystallisation defect in cholelithiasis. Gut. 1995;37:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Groen AK, Stout JP, Drapers JA, Hoek FJ, Grijm R, Tytgat GN. Cholesterol nucleation-influencing activity in T-tube bile. Hepatology. 1988;8:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Gallinger S, Taylor RD, Harvey PR, Petrunka CN, Strasberg SM. Effect of mucous glycoprotein on nucleation time of human bile. Gastroenterology. 1985;89:648-658. [PubMed] |
| 9. | Harvey PR, Upadhya GA, Strasberg SM. Immunoglobulins as nucleating proteins in the gallbladder bile of patients with cholesterol gallstones. J Biol Chem. 1991;266:13996-14003. [PubMed] |
| 10. | Upadhya GA, Harvey PR, Strasberg SM. Effect of human biliary immunoglobulins on the nucleation of cholesterol. J Biol Chem. 1993;268:5193-5200. [PubMed] |
| 11. | Busch N, Lammert F, Matern S. Biliary secretory immunoglobulin A is a major constituent of the new group of cholesterol crystal-binding proteins. Gastroenterology. 1998;115:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Abei M, Kawczak P, Nuutinen H, Langnas A, Svanvik J, Holzbach RT. Isolation and characterization of a cholesterol crystallization promoter from human bile. Gastroenterology. 1993;104:539-548. [PubMed] |
| 13. | de Bruijn MA, Mok KS, Nibbering CP, Out T, Van Marle J, Stellaard F, Tytgat GN, Groen AK. Characterization of the cholesterol crystallization-promoting low-density particle isolated from human bile. Gastroenterology. 1996;110:1936-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Jiao W, Zhang YL, Cai R. [Isolation, purification and the characteristics of 70 KD pronucleation glycoprotein in the bile]. Zhonghua Waike Zazhi. 1994;32:271-274. [PubMed] |
| 15. | Li F, Cai D, Mo HQ, Zhang YL. The ELISA for the 200 kDa glycoprotein in bile. Zhonghua Xiaohua Zazhi. 1997;17:333-335. |
| 16. | Abei M, Schwarzendrube J, Nuutinen H, Broughan TA, Kawczak P, Williams C, Holzbach RT. Cholesterol crystallization-promoters in human bile: comparative potencies of immunoglobulins, alpha 1-acid glycoprotein, phospholipase C, and aminopeptidase N1. J Lipid Res. 1993;34:1141-1148. [PubMed] |
| 17. | Miquel JF, Rigotti A, Rojas E, Brandan E, Nervi F. Isolation and purification of human biliary vesicles with potent cholesterol-nucleation-promoting activity. Clin Sci (. Lond). 1992;82:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Miquel JF, Núñez L, Rigotti A, Amigo L, Brandan E, Nervi F. Isolation and partial characterization of cholesterol pronucleating hydrophobic glycoproteins associated to native biliary vesicles. FEBS Lett. 1993;318:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Ma BJ, Cai D, Zhang QH, Zhang YL. The potent cholesterol-nucleation activity of human biliary vesicles. Zhonghua Gandan Waike Zazhi. 1998;4:104-106. |
| 20. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 188875] [Article Influence: 3434.1] [Reference Citation Analysis (0)] |
| 21. | Guo CX, Guo XQ. Introducing a simple, rapid, and effective method of labeling antibody with peroxidase using periodicacid. Shanghai Mianyixue Zazhi. 1983;2:97-100. |
| 22. | Elder JH, Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci USA. 1982;79:4540-4544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 509] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Plummer TH, Phelan AW, Tarentino AL. Detection and quantification of peptide-N4- (N-acetyl-beta-glucosaminyl)asparagine amidases. Eur J Biochem. 1987;163:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Busch N, Tokumo H, Holzbach RT. A sensitive method for determination of cholesterol growth using model solutions of supersaturated bile. J Lipid Res. 1990;31:1903-1909. [PubMed] |
| 25. | Busch N, Matiuck N, Sahlin S, Pillay SP, Holzbach RT. Inhibition and promotion of cholesterol crystallization by protein fractions from normal human gallbladder bile. J Lipid Res. 1991;32:695-702. [PubMed] |
| 26. | Burnstein MJ, Ilson RG, Petrunka CN, Taylor RD, Strasberg SM. Evidence for a potent nucleating factor in the gallbladder bile of patients with cholesterol gallstones. Gastroenterology. 1983;85:801-807. [PubMed] |
| 27. | Chen Y, Zhang Y, Cai D. [The actions of nucleating proteins in vesicle aggregation and fusion: A preliminary study]. Zhonghua Waike Zazhi. 1997;35:181-185. [PubMed] |
| 28. | Afdhal NH, Niu N, Gantz D, Small DM, Smith BF. Bovine gallbladder mucin accelerates cholesterol monohydrate crystal growth in model bile. Gastroenterology. 1993;104:1515-1523. [PubMed] |
| 29. | Jirsa M, Muchová L, Dráberová L, Dráber P, Smíd F, Kuroki M, Marecek Z, Groen AK. Carcinoembryonic antigen-related cell adhesion molecule 1 is the 85-kilodalton pronase-resistant biliary glycoprotein in the cholesterol crystallization promoting low density protein-lipid complex. Hepatology. 2001;34:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | de Bruijn MA, Mok KS, Out T, Tytgat GN, Groen AK. Immunoglobulins and alpha 1-acid glycoprotein do not contribute to the cholesterol crystallization-promoting effect of concanavalin A-binding biliary protein. Hepatology. 1994;20:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |