Published online Oct 15, 2002. doi: 10.3748/wjg.v8.i5.879
Revised: January 20, 2002
Accepted: January 23, 2002
Published online: October 15, 2002
AIM: To assess and compare the efficacy and safety of two triple regimes: A) metronidazole, amoxicillin and omeprazole, which is still widely used in Russia, and B) azithromycin, amoxicillin and omeprazole in healing active duodenal ulcer and H. pylori eradication.
METHODS: 100 patients with active duodenal ulcer were included in the open, multicentre, randomized study with comparative groups. Patients were randomly assigned to one of the following one-week triple regimes: A) metronidazole 500 mg bid, amoxicillin 1 g bid and omeprazole 20 mg bid (OAM, n = 50) and B) azithromycin 1 g od for the first 3 d (total dose 3 g), amoxicillin 1 g bid and omeprazole 20 mg bid (OAA, n = 50). Omeprazole 20 mg od was given after the eradication course as a monotherapy for three weeks. The control endoscopy was performed 8 wk after the entry. H. pylori infection was determined in the entry of the study and four weeks after the cessation of treatment by means of histology and CLO-test.
RESULTS: 97 patients completed the study according to the protocol (1 patient of the OAM group did not come to the control endoscopy, 2 patients of the OAA group stopped the treatment because of mild allergic urticaria). Duodenal ulcers were healed in 48 patients of the OAM group (96%; CI 90.5%-100%) and in 46 patients of the OAA group (92%; CI 89.5%-94.5%) (p = ns). H. pylori infection was eradicated in 15 out of 50 patients with OAM (30%; CI 17%-43%) and in 36 out of 50 patients treated with OAA (72%; CI 59%-85%) (P < 0.001) - ITT analysis.
CONCLUSION: The triple therapy with omeprazole, amoxicillin and metronidazole failed to eradicate H. pylori in the majority of patients, which is an essential argument to withdraw this regimen out of the national recommendations. Macrolide with amoxicillin are preferable to achieve higher eradication rates. Azithromycin (1 g od for the first 3 d) can be considered as a successful component of the triple PPI-based regimen.
-
Citation: Ivashkin VT, Lapina TL, Bondarenko OY, Sklanskaya OA, Grigoriev PY, Vasiliev YV, Yakovenko EP, Gulyaev PV, Fedchenko VI. Azithromycin in a triple therapy for
H.pylori eradication in active duodenal ulcer. World J Gastroenterol 2002; 8(5): 879-882 - URL: https://www.wjgnet.com/1007-9327/full/v8/i5/879.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i5.879
A number of antimicrobial agents have been used in various regimens to eradicate Helicobacter pylori. The properties of different medications may have some impact on the therapy result. Clinical trails are undertaken to search for simpler but equally effective (or more effective) regimen. The modern macrolides are in the focus of attention from that point of view. Azithromycin, a new generation macrolide, has some special attributes, that makes it a promising compound in the regimens for H. pylori eradication. It is acid-stable, has a long half-life and achieves remarkably high concentration in the gastric tissue. Thus after a single oral dose of 500 mg the concentration of azithromycin persisted in the gastric mucosa above the MIC90 for H. pylori over a five-day period[1]. There were several clinical trails with azithromycin in the therapy of H. pylori infection. As pharmacological properties of azithromycin make possible to use shorter courses, the problem was to define an optimal dose and duration of azithromycin in the triple therapy. The triple regimens with the total course dose of azithromycin of 1.5 g gave high eradication rates[2,3], but the result was not stable[4], the total course dose of 3 g appeared to be more reliable[5,6].
There were just a few studies of azithromycin in the treatment of H. pylori infection in peptic ulcer and chronic gastritis in Russia[7,8]. The results were satisfactory, and it was clear that further studies to reach the level of evidence-based medicine are needed.
The clinical trial of azithromycin (Sumamed®, PLIVA) in the triple regimen for the eradication of H. pylori in active peptic ulcer, that was planned according to the GCP criteria, was of priority significance for Russia. The aim of the study was to assess and compare the efficacy of two triple regimes: (A) omeprazole, amoxicillin and metronidazole, and (B) omeprazole, amoxicillin and azithromycin in healing active duodenal ulcer and H. pylori eradication. The safety and tolerability of the two drug combinations were also evaluated and compared.
It was an open, randomized study with comparative groups conducted in three Moscow gastroenterological centers: V. Vasilenko Clinic of internal deseases, gastroenterology and hepatology of the Moscow Sechenov Medical Academy, Gastroenterological Center of the Health Ministry of Russia and Central Institution of Gastroenterology. The study protocol was worked out by PLIVA pharmaceutical company (Zagreb, Croatia).
The study was conducted according to GCP guidelines and the Helsinki Declaration. All patients gave written informed consent and the protocol was approved by the local Ethic Committees of the above-mentioned centers.
Out-patients and in-patients of both sexes aged between 18 and 70 years with endoscopically proven one or more duodenal ulcers were eligible for entry into the study. H. pylori presence before the treatment was detected by a rapid urea test and histology. In the CLO-test (Delta-West Ltd, Australia) two biopsy specimens (one from the antrum and one from the corpus on the greater curvature) were examined. The positive result of the CLO-test was needed to involve the patient into the study. Four biopsy specimens (two from the antrum and two from the mid-corpus on the greater and lesser curvature) underwent histopathological assessment. Sections of paraffin-embedded specimens were routinely stained with haematoxylin-eosin for morphologic examination and with Giemsa for H. pylori detection.
The information about the study was accessible to all patients before the entry. The written consent was necessary for participation in the clinical trial. The criteria for exclusion were: intake of proton pump inhibitors, antibiotic or bismuth salts within 4 wk prior to the study, ulcer complications, concomitant gastric ulcer or reflux oesophagitis of grade II or more according to the classification of Savari et Miller, stomach surgery (except for a simple closure of perforation), known hypersensitivity to one of the study medications, severe concomitant diseases with metabolic changes, suspected poor compliance. Patients were required to be a male or nonpregnant, nonlactating females; females were postmenopausal or using a contraceptive.
At the entry the patients had a full physical examination. Routine haemotological and biochemical (serum creatinine, urea, transaminases, alkaline phosphatase, total and direct bilirubine) screening was carried out.
The patients, that satisfied the inclusion criteria, were randomly assigned to one of the following one-week triple regimes:
(A) metronidazole 500 mg bid, amoxicillin 1 g bid and omeprazole (Losec®, AstraZeneca) 20 mg bid (OAM) and (B) azithromycin (Sumamed®, PLIVA) 1 g od for the first 3 d (total dose 3 g), amoxicillin 1 g bid and omeprazole (Losec®, AstraZeneca) 20 mg bid (OAA).
Omeprazole (Losec®, AstraZeneca) 20 mg od was given after the eradication course as a monotherapy for three weeks.
Control examination was performed 4 wk after the cessation of omeprazole monotherapy (8 wk after entry). Physical status, adverse events, haemotological and biochemical analysis, endoscopy (ulcer healing) were assessed. H. pylori infection was determined by histology and CLO-test: cure of the infection was established if two tests of all biopsy specimens gave negative results (two from the antrum, two from the corpus on the greater and lesser curvature for histology and one from the antrum, one from the corpus on the greater curvature for CLO-test).
The duodenal ulcer healing rates and H. pylori eradication rates were compared between the two treatment groups using a χ²-test. A two-sided 95% confidence interval (95%CI) was calculated using the normal approximation to the binominal distribution.
100 patients entered the trial: 50 patients were randomized to group A, and 50-to group B. The two treatment groups had similar demographic characteristics.
The patients at the entry usually had the dyspeptic symptoms typical for active duodenal ulcer. The ulcers localized in the duodenum bulb were between 0.3 cm and 1.5 cm in size (the two thirds of patents had ulcers between 0.5 cm and 1.0 cm in size). Three patients with two duodenal ulcers were randomized to group B.
49 patients (out of 50) of group A (metronidazole, amoxicillin and omeprazole) completed the study without contravention to the protocol: one patient was lost for the follow-up. Two patients of group B (azithromycin, amoxicillin and omeprazole) had mild allergic symptoms (urticaria) in the beginning of the eradication course and stopped treatment. A short use of antihistamine medications led to relief of allergy. Thus, 48 patients of group B (out of 50) completed the study according to the protocol.
The efficacy of treatment regimens was assessed by duodenal ulcer healing. Endoscopy performed 4 wk after the cessation of omeprazole monoterapy revealed that duodenal ulcers healed in 48 patients of the OAM group and in 46 patients of the OAA group. The causes of ulcer persistence were failed H. pylori eradication and drop-out from the protocol. A patient with missing data from group A and two patients of group B (drop-outs due to adverse events) were included in the analysis for ulcer healing as "not healed" Ulcer healing rate in group A was 96% (CI 90.5%-100%) and in group B 92% (CI 89.5%-94.5%). Statistical difference was not significant.
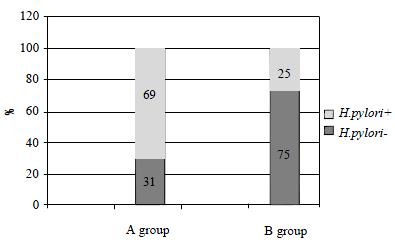
The main indicator of the triple regimen effectiveness was the rate of H. pylori eradication. Eradication rates were estimated for the population that completed the study according to the protocol (per protocol analysis) and for the population that was involved in the study (intention-to-treat analysis). One patient of group A, that did not come to the control examination, and two patients of group B, withdrawn from the study because of the adverse events, were estimated as a negative eradication result. H. pylori infection was eradicated in 15 out of 50 patients with OAM: eradication rate was 30.6% (95%CI: 17.6%-43.6%) PP and 30% (95%CI: 17%-43%) ITT. H. pylori infection was eradicated in 36 out of 50 patients treated with OAA: eradication rate was 75% (95%CI: 63%-87%) PP and 72% (95%CI: 59%-85%)-ITT analysis (Figure 1).
The difference between the treatment groups was statistically significant: P < 0.001.
The treatment safety was assessed by adverse events recording, the laboratory tests deviation of clinical significance were also taken into consideration. The two cases of withdrawal because of an allergic urticaria were registered in group B. Quick relief of the allergic symptoms due to the antihistamine preparations allowed us to consider this adverse events as mild. The laboratory parameters of haemotological and biochemical tests (serum creatinine, urea, transaminases, alkaline phosphatase, total and direct bilirubine) were usually normal. There was no clinically significant changes from the baseline in the laboratory results.
The new triple regime for H. pylori eradication with new macrolide azithromycin was compared with the combination of metronidazole, amoxicillin and omeprazole, which is still widely used in Russia.
Both combinations were highly effective in ulcer healing. The analysis of cases with persistent duodenal ulceration revealed that most of them were connected with protocol nonfulfilment and failed eradication. High healing rates are expected results of triple therapies based on proton pump inhibitors. Both proton pump inhibitors action and the effect of H. pylori eradication are of importance in ulcer healing. Persistence of H. pylori infection in gastric mucosa is considered now as one of the factors preventing ulcer healing, or to be more precise, as a factor of distortion of the normal regeneration process[9]. This molecular events are not so evident in every-day clinical practice as potent effect of omeprazole. Proton pump inhibitors-based triple therapies even without "posteradicational" antisecretory monotherapy are advantageous in active duodenal ulcer. Rapid symptoms relief and ulcer healing in 96% patients of group A and in 96% patients of group B (per protocol) once more proved the effectiveness of eradication therapy based on omeprazole. Rapid ulcer healing did not depend on antibiotic composition.
There are no therapies that eradicate H. pylori infection in every case. That is why the search for better anti-H. pylori regimes is of present interest. Clinical experience of the Russian trials that are organized according to the principals of evidence-based medicine is of great importance. They give information about the possibility of application of foreign data to the Russian patient population. The therapy used in group A "metronidazole 500 mg bid, amoxicillin 1 g bid and omeprazole 20 mg bid" is widely used in the Russian practice. This regime is well-known to general practitioners and is included in "The guidelines for the management of Helicobacter pylori infection" of the Russian Helicobacter pylori Study Group and Russian Gastroenterological Association, adopted in 1997[10]. Since that time there were some reports that informed about very low eradication rates with proton pump inhibitor, metronidazole and amoxicillin[11,12]. The present clinical trial demonstrated (by the example of the patients from three Moscow gastroenterological centers) discouraging eradication rate in the OAM triple therapy. This is a real argument in favour of withdrawing regime "proton pump inhibitor, metronidazole and amoxicillin" from the national guidelines as it was done in the Maastrcht 2-2000 European Consensus report[13].
Antimicrobial resistance of H. pylori strains is one of the main causes of treatment failure. H. pylori resistance to nitroimidazoles (metronidazole and tinidazole) is quite an often event in the Russian populations. Thus, in Moscow H. pylori strains with primary metronidazole resistance were found in more than 50% of isolates[14]. Unsuccessful anti-H. pylori course of metronidazole- containing regimen usually leads to secondary resistance. One-third of the patients in the present study had a long duration of peptic ulcer disease (> 5 years), almost all of them had used metronidazole. H. pylori susceptibility testing was not considered in the present trial, but we could suspect, with high probability, nitroimidazole resistance as a cause of eradication failure in two-thirds of the group A patients.
The proton pump inhibitor-based triple therapy with amoxicillin and clarithromycin gives steady high eradication rates. The European clinical trial MACH1 demonstrated the best result using omeprazole 40 mg with amoxicillin 1 g bid and clarithromycin 500 mg bid among five omeprazole-based combinations with different antimicribials for seven days[15]. The proton pump inhibitor (or ranitidine bismuth citrate) in combination with clarithromycin 500 mg bid and amoxicillin 1 g bid were named the preferable regimen for first-line eradication therapy in the Maastrcht-2 Consensus report[13]. The real chance to enhance the eradication rates in the countries with high levels of metronidazole resistance is to avoid metronidazole in the anti-H. pylori treatment. Provided the levels of clarithromycin resistance are low the macrolide and amoxicillin regimens would be the most beneficial.
The treatment regimen of group B "azithromycin 1 g od for the first 3 d, amoxicillin 1 g bid and omeprazole 20 mg bid for 7 d" eradicated H. pylori infection in 72% of patients, the result is good for the Russian populations. This rate of H. pylori eradication seems to be lower that is acceptable. But according to some publications it is quite common with accepted PPI-based triple therapies. Thus J.P. Gisbert et al[16]. gave mean eradication rate (weighted mean) as 71% for ITT analysis for 7-day to 14-day omeprazole-based therapies.
H. pylori has cross resistance to macrolides: the strain resitant e.g. to clarithromycin is resistant to every other macrolide. The level of clarithromycin resistance in Moscow is 8%-14%, unfortunately with the tendency for an increase[14]. The effect of drug synergism is of great value in combination treatment to heal H. pylori infection. P.M. Lepper et al[17]. demonstrated in vitro synergistical effect of azithromycin and proton pump inhibitor lansoprazole. They speculate that this effect may enhance eradication rates even with macrolide-resistant H. pylori strains because of the unique pharmacological properties of the combination. Azithromycin could provide a potent anti-H. pylori effect and could simplify the bulky triple therapy. Of macrolides azithromycin develops the highest concentration in gastric tissue and mucus, its pharmacokinetic properties makes it possible to take azithromycin only once a day and only during three days in a week course. Clarithromycin for standard eradication is administered for 7 d twice a day (usually 4 tablets of 250 mg). Azithromycin is really an advantageous medication to reach simpler therapy, improving both tolerability and compliance. The correctness of the azithromycin dose chosen in our trial -1 g daily for three days - was confirmed by recent results[18].
In conclusion, we have shown that azithromycin has clinical (in vivo) activity against H. pylori infection. Azithromycin (1 g od for the first 3 d in a week course) can be considered as a successful component of the triple proton pump inhibitor-based regimen. It is necessary to eradicate H. pylori in peptic ulcer patients, using effective and simple regimens.
Edited by Xia HHX
| 1. | Harrison JD, Jones JA, Morris DL. Azithromycin levels in plasma and gastric tissue, juice and mucus. Eur J Clin Microbiol Infect Dis. 1991;10:862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Bertoni G, Sassatelli R, Nigrisoli E, Tansini P, Bianchi G, Della Casa G, Bagni A, Bedogni G. Triple therapy with azithromycin, omeprazole, and amoxicillin is highly effective in the eradication of Helicobacter pylori: a controlled trial versus omeprazole plus amoxicillin. Am J Gastroenterol. 1996;91:258-263. [PubMed] |
| 3. | Labenz J, Tillenburg B, Stolte M. Azithromycin as a substitute for clarithromycin in French triple therapy for Helicobacter pylori: a randomized study (abstr.). Gut. 1999;45:A115. |
| 4. | Di Mario F, Dal Bó N, Grassi SA, Rugge M, Cassaro M, Donisi PM, Vianello F, Kusstatscher S, Salandin S, Grasso GA. Azithromycin for the cure of Helicobacter pylori infection. Am J Gastroenterol. 1996;91:264-267. [PubMed] |
| 5. | Vcev A, Stimac D, Vceva A, Takac B, Pezerovíc D, Ivandíc A. High dose omeprazole plus amoxicillin and azithromycin in eradication of Helicobacter pylori in duodenal ulcers. Helicobacter. 1999;4:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Chey WD, Fisher L, Barnett J, Delvalle J, Elta GH, Hasler WL, Nostrant T, Palaniappan J, Scheiman J. Low- versus high-dose azithromycin triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Loginov AS, Vasiliev YV. The complex use of azithromycin (Sumamed), amoxicillin and metronidazole in Helicobacter pylori eradication. Rus Gastroenterol J. 1998;3:35-41. |
| 8. | Vasiliev YuV. Sumamed and new perspectives in rational Helicobacter pylori eradication in peptic ulcer and gastritis. Moscow Med J. 1999;6:19-21. |
| 9. | Aruin LI, Kapuller LL, Isakov VA. Morphological diagnostics of gastrointestinal diseases. Moscow: Triada-X 1998; 174-182. |
| 10. | The guidelines for the management of Helicobacter pylori infection in peptic ulcer disease in adult population. Rus J Gastroenterol Hepatol Coloproctol. 1998;1:105-107. |
| 11. | Kurilovich SA, Shlykova LG, Kopychko TA. Real problems of H. pylori eradication (abstr.). Rus J Gastroenterol Hepatol Coloproctol. 2000;5:25. |
| 12. | Bondarenko OY, Ivashkin VT, Lapina TL, Sklanskaya OA, Charikova SYu. Efficacy of Helicobacter pylori treatment, based on Lansoprazole produced in Russia. Siberian J Gastroenterol Hepatol. 2000;10:10-11. |
| 13. | Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 843] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 14. | Koudryavtseva LV, Isakov VA, Ivanikov IO, Zaitseva SV. Evolution of H. pylori primary rasistance to antimicrobial agents in Moscow (Russia) in 1996-abstr.. Gut. 2000;47 (Suppl.):A8. |
| 15. | Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdörffer E, O'Morain C, Bardhan KD, Bradette M, Chiba N, Wrangstadh M. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996;1:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 394] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Gisbert GP, Pajares JM, Racz I. Therapy. Current Opinion Gastroenterol. 2001;17:S47-S54. |
| 17. | Lepper PM, Moricke A, Glasbrenner B, Trautman M. Demonstration of in-vitro synergism between proton-pump inhibitors and macrolides against Helicobacter pylori (abstr.). Gut. 2000;47:A110. |
| 18. | Bazzoli F, Zagari R, Albanese R, Pozzato P, Fossi S, Berretti D, Martuzzi C, Lunedei V, Antonini F, Roda E. Three days 1000 vg vs 500 mg daily Azithromycin with tinidazole and omeprazole for Helicobacter pylori eradication: a double blind randomized, placebo controlled multicenter study (abstr.). Gut. 2001;49:A84. |