Published online Oct 15, 2002. doi: 10.3748/wjg.v8.i5.827
Revised: July 1, 2002
Accepted: July 6, 2002
Published online: October 15, 2002
AIM: To study the effects of doxorubicin on telomerase activity and telomere length in hepatocellular carcinoma.
METHODS: Telomerase activity was assayed with a non-radioisotopic quantitative telomerase repeat amplification protocal-based method. The effect of doxorubicin (DOX) on the growth of BEL-7404 human hepatoma cells was determined by microculture tetrazolium assay. Mean telomere length (terminal restriction fragment) was detected by Southern blot method. The expression of telomerase subunits genes was investigated by RT-PCR. Cell apoptosis and cell cycle distribution were evaluated by flow cytometry.
RESULTS: Telomerase activity was inhibited in a dose and time-dependent manner in BEL-7404 human hepatoma cells treated with DOX for 24, 48 or 72 h in concentrations from 0.156 to 2.5 μM which was crrelated with the inhibition of cell growth. No changes were found in the mRNA expression of three telomerase subunits (hTERT, hTR and TP1) after drug exposure for 72 h with indicated concentrations. The cells treated with DOX showed shortened mean telomere length and accumulated at the G2/M phase. However, there was almost no effects on cell apoptosis by DOX.
CONCLUSION: The telomerase inhibition and the telomere shortening by DOX may contribute to its efficiency in the treatment in hepatocellular carcinoma.
- Citation: Zhang RG, Guo LX, Wang XW, Xie H. Telomerase inhibition and telomere loss in BEL-7404 human hepatoma cells treated with doxorubicin. World J Gastroenterol 2002; 8(5): 827-831
- URL: https://www.wjgnet.com/1007-9327/full/v8/i5/827.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i5.827
Telomeres form the ends of eukaryotic chromosomes consisting of an array of tandem repeats of hexanucleotide 5'-TTAGGG-3'. Telomeres protect the chromosomes from DNA degradation, end-to-end fushions, rearrangements and maintain nuclear structure[1]. Human telomerase is a ribonucleoprotein complex, composed of a catalytic reverse transcriptase subunit (hTERT), an RNA component (hTR) that serves as a template for the synthesis of telomeric repeats, and an associated protein subunit (TP1)[2-4]. It adds telomeric repeats to the 3'end of telomeric DNA. This telomere stabilization by telomerase can lead to unlimited cell proliferation.
Hepatocellular carcinoma (HCC), one of the most common malignancies in the world especially in Asia and Africa, is an aggressive cancer. It causes approximately 250000 deaths annually[5]. It was reported that HCC exhibited a high incidence of telomerase activity and that the activity increased in accordance with the HCC degree of histological undifferentiaton which was absent in normal liver tissue[6,7]. Other reports revealed that hTERT expression was the rate-limiting determinant of HCC telomerase activity[8-10].
Doxorubicin (DOX), an antitumor antibiotic, can intercalate into base pairs of DNA and generate toxic oxygen free radicals, which not only causes single-or double-strand DNA breaks but also damages a variety of necessary macromolecules such as proteins, lipids and RNA[11]. DOX is one of the most efficient chemotherapy agents in the treatment of HCC, and its total efficiency rate can be up to 44%[12]. However, the relationship between the efficiency of DOX and telomerase activity in HCC has not yet been elucidated. In the present study, we investigated the effects of DOX on the telomerase activity and telomere length in BEL-7404 human hepatoma cells.
BEL-7404 human hepatoma cell line from Cell Bank of Chinese Academy of Sciences[13], was cultured in RPMI-1640 medium (Gibco) supplemented with 10% heat-inactivated newborn calf serum, at 37 °C in a humidified CO2 incubator containing 5% CO2 and 95% air.
DOX (Sigma) was dissolved in RPMI-1640 to the final concentration of 5 mM and stored at 4 °C.
An MTT assay was conducted to determine the cell proliferation. Cells were seeded at 1 × 104 cells/well in a 96-well plate and incubated overnight. The drug was added to the cultured cells with the final concentrations from 0.156 μM to 2.5 μM and culturing further for another 24, 48 or 72 h respectively. Following culture, the cells were incubated with 800 mg/L 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma), which was used to assay the activity of mitochondrial dehydrogenases. Four hours later, 10% sodium dodecyl sulphate - 5% isobutanol- 0.12% hydrochloric acid solution was added to solubilize the formazan product. The plate was then incubated at 37 °C for another 12 h. The absorbance at 570 nm was measured with a model 550 microplate reader (Bio-Rad). The percent of cell growth inhibition was expressed as: (A-B)/A × 100%, where A was the absorbance value from the controls and B was that from the experimental cells.
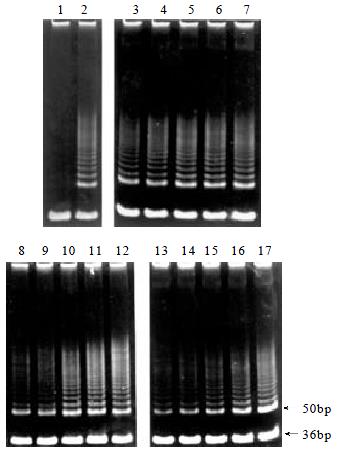
Telomerase activity was assayed with PCR-based telomeric repeat amplification protocol (TRAP) as previously described[14,15]. Cells were collected and washed with PBS, lysed in 1 × 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonic acid (CHAPS, Sigma) buffer, incubated on ice for 30 min, and centrifuged at 12000 g for 30 min. The protein concentration was determined by Coomassie Protein Assay. Each of TRAP reactions contained 1 μg of total protein. The reaction mixture [20 mM Tris-HCl (pH8.3), 1.5 mM MgCl2, 63 mM KCl, 0.005% Tween-20, 1 mM EGTA, 50 μM of each dNTPs and 0.1 μg TS (5'-AATCCGTCGAGC AGAGTT-3'] was incubated at 30 °C for 30 min, heated at 94 °C for 5 min. Then 0.1 μg of return primer ACX (5'-GCGCGG[CTTACC]3CTAACC-3', 0.1 μg of internal control primer NT (5'-ATCGCTTCTCGGCCTTTT-3', 0.01 mM of internal control template TSNT (5'-AATCCGTCGAGCAGAGTTAAAAGGCCGAGAAGCGAT-3' and 2 units Taq DNA polymerase (Promega) were added. The reaction mixture was then subjected to 28 PCR cycles: 94 °C for 30 s and 60 °C for 30 s. PCR products were separated by electrophoresis on 12% nondenaturing polyacrylamide gels and stained with SYBR Green I (FMC) for 15 min, visualized and analyzed by UVP system. In every experiment, a negative control (1 μL CHAPS lysis buffer) was included. All of experiments were repeated at least twice. The relative telomerase activity was quantified by the formula:
TP = [(A/B)/(A cell control/B cell control)] × 100
Where TP = total product, A = total intensity of telomerase product (50 bp, 56 bp, 62 bp..., and B = intensity of internal control (36 bp).
Total cellular RNA was extracted from cells using Trizol (Life Technologies, Inc.) according to the instructions of the manufacturer. In each reaction, 1 μg of total RNA was reverse transcripted into cDNA using M-Mlv reverse transcriptase (Promega). Primer sets used to amplify specific sequences were 5'-CGGAAGAGTGTCTGGAGCAA-3' and 5'-GGATGAAGCGGAGTCGGA-3' for hTERT (146 bp); 5'-TCTAACCCTAACTGAGAAGGGCGTAG-3' and 5'-GTTTGCTCTAGAATGAACGGTGGAAG-3' for hTR (126 bp); 5'-TCAAGCCAAACCTGAATCTGAG-3' for TP1 (264 bp); 5'-GTGGGGCGCCCCAGGCACCA-3' and 5'-GTCCTTAATGTCACGCACGATTTC-3' for β-actin (539 bp). The PCR conditions of hTERT and TP1 were 94 °C, 45 s; 60 °C, 45 s; 72 °C, 90 s for 31 and 29 cycles, respectively. And the PCR conditions of hTR and β-actin were 94 °C, 45 s; 55 °C, 45 s; 72 °C, 90 s for 28 and 22 cycles, respectively[2].
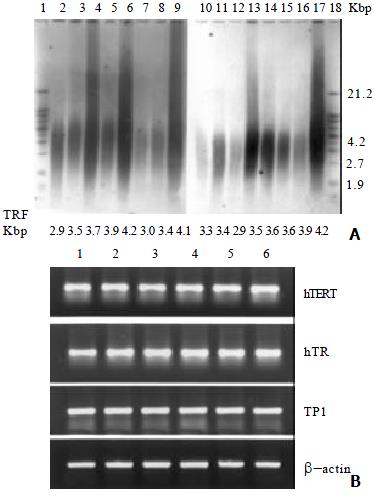
Genomic DNA samples were prepared as described[16]. Cells were lysed and proteins were digested in 10 mM Tris-HCl (pH8.0), 100 mM NaCl, 25 mM EDTA, 0.5% SDS, 0.1 mg/mL proteinase K at 48 °C overnight. Following two extractions with phenol and one with chloroform, DNA was precipitated with ethanol and dissolved in 10 mM Tris-HCl (pH8.0) and 1 mM EDTA (TE) Telomere length was detected using TeloTAGGG telomere length assay (Roche) according to the manufacturer’sprotocol. For each sample, 1 μg of genomic DNA was digested with Rsa I/Hinf I (Sigma), separated on a 0.8% agarose gel, transferred to a nylon membrane (Amersham Hybond-N+), and hybridized with a telomere specific digoxigenin (DIG)-labeled probe, incubated with anti-DIG-alkaline phosphatase and detected by chemiluminescence. The blotted signal was divided into 30 equidistant intervals from 1.9 to 21.2 kilobases to calculate mean telomere length (terminal restriction fragment, TRF) using the formula TRF = Σ(ODi)/Σ(ODi/Li), where ODi was the chemiluminescent signal and Li was the length of the TRF fragment at positioni[17].
The cells were harvested and resuspended in the solution containing 40 mM sodium citrate, 250 mM sucrose and 5% DMSO. The suspention was stored at -20 °C for 20 min, then thawed rapidly at room temperature and centrifuged to collect the cells. The cells were resuspended in a solution containing RNase A (5 × 104 unit/g, 50 mg/L) and 20 mg/L propidium iodide (PI). The cell cycle distribution and apoptosis were determined by the fluorescence of individual cells measured with flow cytometry[18].
Telomerase activity was inhibited in a dose and time-dependent manner in BEL-7404 human hepatoma cells treated with DOX (Figure 1A). To analyze the telomerase inhibition on the gene expression level, cells treated with DOX for 72 h were employed to study the telmerase mRNA expressions of its three major gene components, hTERT, hTR and TP1, using RT-PCR. Results showed that no changes were observed in the mRNA expression pattern of these subunits after the DOX exposure for 72 h with indicated concentrations (Figure 1B).
The first report on the telomerase inhibition by DOX was presented by Zhu et al[19], regarding the SW480 colon carcinoma cells. However, the opposite observations were reported later, in which authors found no effect of DOX on telomerase regulation in nasopharyngeal cancer, testicular cancer and squamous cell carcinoma[20-22]. In this study, we found that the DOX treatment inhibited the telomerase activity in a dose and time-dependent manner in BEL-7404 human hepatoma cells. Considering the different results above, We think that this kind of inhibition might be cell-type specific and may be correlated with the clinical different efficiency of DOX in the treatment of different kinds and stages of tumors.
Abundant evidence indicated that the regulation of telomerase was multifactorial in mammalian cells and involves telomerase gene expression, post-translational protein-to-protein interactions, and protein phosphorylation[23]. However, there was no information regarding three telomerase subunits expression affected by DOX in Zhu's study[18]. In our investigation, there were no changes found in the expression of hTERT, hTR or TP1 mRNA. This indicated that the telomerase inhibition by DOX might be indirect with its antibiotic activity (e.g. J damaging of necessary macromolecules).
Mean telomere length of BEL-7404 human hepatoma cells was decreased by the DOX treatment (Figure 2). Here, we believe that this is the first report regarding the shortening of telomere length by DOX treatment in BEL-7404 human hepatoma cells.
Compared with normal cells and some other carcinoma cells (such as Hela cells), the mean telomere length of BEL-7404 human hepatoma cells is relatively short. As a result, the hepatoma cells are sensitive to telomere shortening, which may account, at least in part, for their chemosensitivity. Results from telomere shortening by cisplatin indicate that the nucleotide-excision repair system and cell division dynamics might be involved in the telomere shortening process, which make the process showing in a non- dose or time dependent manner[24]. We assume that both of the factors may likewise participate in telomere shortening by DOX, because DOX can intercalate into DNA base pairs and result in non-dose or time dependent manner of telomere shortening.
Accumulation of cell cycle in G2/M phase
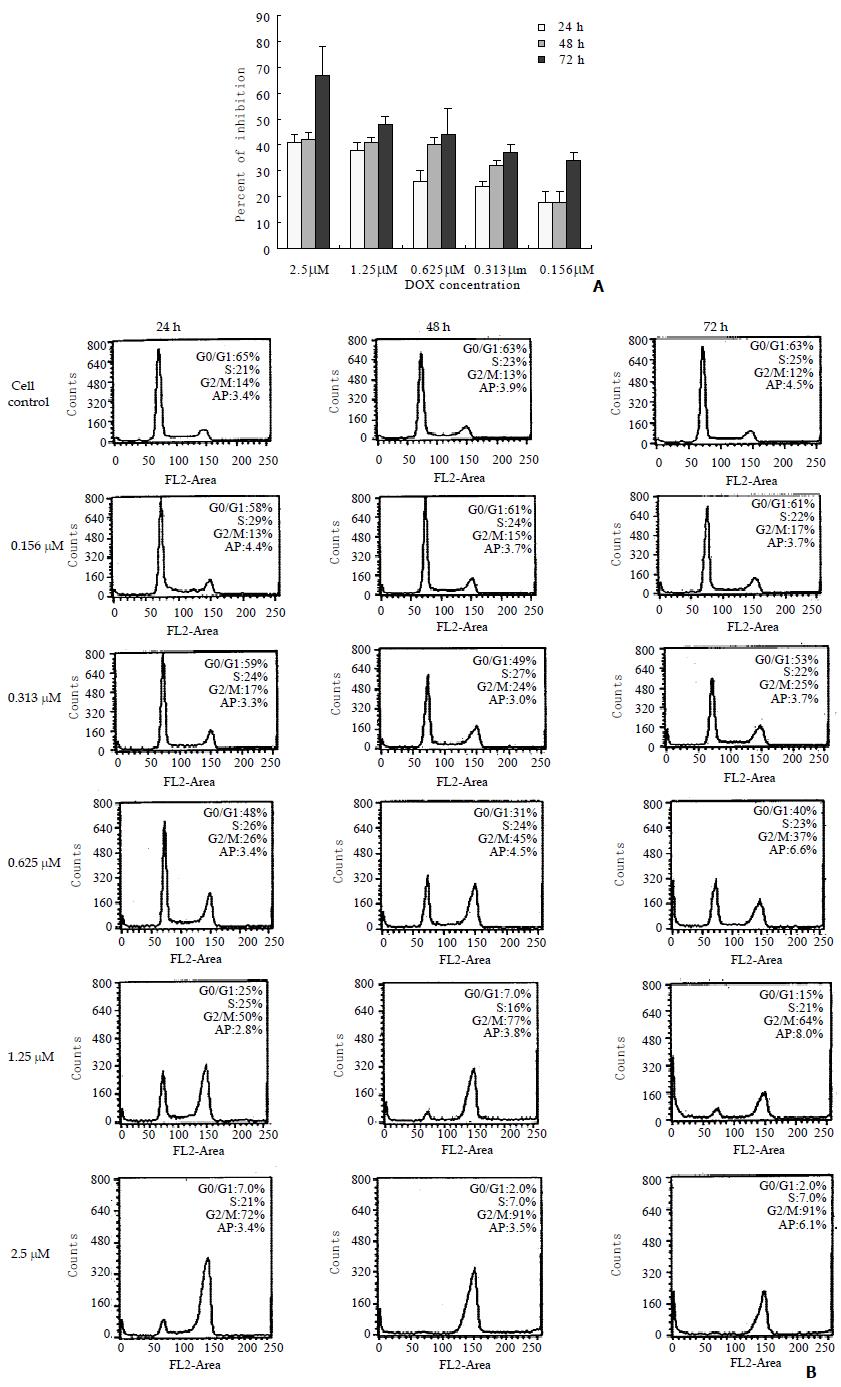
The cell growth inhibition and cell cycle progression during DOX treatment were monitored in the present study to correlate these effects with telomerase activity inhibition and telomere loss. The growth of BEL-7404 cells was inhibited by DOX, which was indicated in (Figure 3A). Following exposure to DOX, the hepatoma cells were accumulated in G2/M phase, which revealed by flow cytometry (Figure 3B). There were no marked changes observed in cell apoptosis in the experimental cells exposure to DOX for 24, 48 or 72 h with the concentrations from 0.156 to 2.5 μM, compared with the control cell.
Zhu et al[19] attributed the reduction in telomerase activity by DOX to the accumulation of cell cycle in G2/M phase. But Holt et al[25] found that telomerase inhibition did not correlate with the cell cycle arrested at G2/M phase but with the increasing in cell death. In present study, the cell apoptosis did not increase markedly compared with the control. Our previous research has also showed that antisense oligonucleotide to telomerase RNA component accumulated the cell cycle in G2/M phase[26]. The present observation confirmed the reports above, which indicated that telomerase inhibition was correlated with the cell cycle arrested at G2/M phase.
Previously, Ishibashi et al[24] have reported that telomere loss in HeLa cells associated with the apoptosis inducted by cisplatin. However, in our investigation the percent of cell apoptosis did not change markedly (Figure 3 B), although the mean telomere length was reduced by DOX. In BEL-7404 human hepatoma cells, we previously found that the cell apoptosis occurred when the mean telomere length reached to about 1.7 Kb[27]. In this study, the mean telomere did not reach to this critical length, and the apoptosis had not been induced which was consistent with the previous observation. Therefore, we deduce that telomere shortening may not be correlated with the apoptosis.
Many studies have shown that the telomerase activity was correlated with the cell growth[28]. Our finding also revealed a good correlation between the inhibition of telomerase activity and the reduction in cell growth. However, in this study, the cell growth inhibition was mainly the result of cell cycle arrest, but not the increasing of cell apoptosis.
In conclusion, we found that telomerase activity was inhibited in a dose and time - dependent manner and mean telomere length was decreased by the treatment of DOX in BEL-7404 human hepatoma cells. This process correlated with the cell growth inhibition and the cell cycle accumulation in G2/M phase. The present study indicates that the telomerase inhibition and the telomere shortening by DOX may contribute to its efficiency in the treatment of HCC.
Edited by Zhu L
| 1. | Yakoob J, Hu GL, Fan XG, Zhang Z. Telomere, telomerase and digestive cancer. World J Gastroenterol. 1999;5:334-337. [PubMed] |
| 2. | Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1680] [Cited by in RCA: 1660] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 3. | Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1606] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 4. | Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 476] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Wang XW, Yuan JH, Zhang RG, Guo LX, Xie Y, Xie H. Antihepatoma effect of alpha-fetoprotein antisense phosphorothioate oligodeoxyribonucleotides in vitro and in mice. World J Gastroenterol. 2001;7:345-351. [PubMed] |
| 6. | Tahara H, Nakanishi T, Kitamoto M, Nakashio R, Shay JW, Tahara E, Kajiyama G, Ide T. Telomerase activity in human liver tissues: comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res. 1995;55:2734-2736. [PubMed] |
| 7. | Nakashio R, Kitamoto M, Tahara H, Nakanishi T, Ide T, Kajiyama G. Significance of telomerase activity in the diagnosis of small differentiated hepatocellular carcinoma. Int J Cancer. 1997;74:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Kawakami Y, Kitamoto M, Nakanishi T, Yasui W, Tahara E, Nakayama J, Ishikawa F, Tahara H, Ide T, Kajiyama G. Immuno-histochemical detection of human telomerase reverse transcriptase in human liver tissues. Oncogene. 2000;19:3888-3893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Takahashi S, Kitamoto M, Takaishi H, Aikata H, Kawakami Y, Nakanishi T, Shimamoto F, Tahara E, Tahara H, Ide T. Expression of telomerase component genes in hepatocellular carcinomas. Eur J Cancer. 2000;36:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Toshikuni N, Nouso K, Higashi T, Nakatsukasa H, Onishi T, Kaneyoshi T, Kobayashi Y, Kariyama K, Yamamoto K, Tsuji T. Expression of telomerase-associated protein 1 and telomerase reverse transcriptase in hepatocellular carcinoma. Br J Cancer. 2000;82:833-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Liu XL, Xiao B, Yu ZC, Guo JC, Zhao QC, Xu L, Shi YQ, Fan DM. Down-regulation of Hsp90 could change cell cycle distribution and increase drug sensitivity of tumor cells. World J Gastroenterol. 1999;5:199-208. [PubMed] |
| 12. | Han R. Primary hepatocellular carcinoma. In Chemoprevention and drug therapy of cancer. Peking: Peking Med University Press 1991; 591-592. |
| 13. | Wang XW, Xie H. Presence of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells. World J Gastroenterol. 1998;4:540-543. [PubMed] |
| 14. | Zhang RG, Wang XW, Yuan JH, Guo LX, Xie H. Using a non-radioisotopic, quantitative TRAP-based method detecting telomerase activities in human hepatoma cells. Cell Res. 2000;10:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR, Wang JP, Zheng ZQ, Wang L. Telomerase activity in gastric cancer and its clinical implications. World J Gastroenterol. 1999;5:316-319. [PubMed] |
| 16. | Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921-1929. [PubMed] |
| 17. | Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Tissue-specific alternate splicing of human telomerase reverse transcriptase (hTERT) influences telomere lengths during human development. Int J Cancer. 2001;91:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Yuan JH, Zhang RP, Zhang RG, Guo LX, Wang XW, Luo D, Xie Y, Xie H. Growth-inhibiting effects of taxol on human liver cancer in vitro and in nude mice. World J Gastroenterol. 2000;6:210-215. [PubMed] |
| 19. | Zhu X, Kumar R, Mandal M, Sharma N, Sharma HW, Dhingra U, Sokoloski JA, Hsiao R, Narayanan R. Cell cycle-dependent modulation of telomerase activity in tumor cells. Proc Natl Acad Sci USA. 1996;93:6091-6095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Ku WC, Cheng AJ, Wang TC. Inhibition of telomerase activity by PKC inhibitors in human nasopharyngeal cancer cells in culture. Biochem Biophys Res Commun. 1997;241:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Burger AM, Double JA, Newell DR. Inhibition of telomerase activity by cisplatin in human testicular cancer cells. Eur J Cancer. 1997;33:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Mese H, Ueyama Y, Suzuki A, Nakayama S, Sasaki A, Hamakawa H, Matsumura T. Inhibition of telomerase activity as a measure of tumor cell killing by cisplatin in squamous cell carcinoma cell line. Chemotherapy. 2001;47:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Liu JP. Studies of the molecular mechanisms in the regulation of telomerase activity. FASEB J. 1999;13:2091-2104. [PubMed] |
| 24. | Ishibashi T, Lippard SJ. Telomere loss in cells treated with cisplatin. Proc Natl Acad Sci USA. 1998;95:4219-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Holt SE, Aisner DL, Shay JW, Wright WE. Lack of cell cycle regulation of telomerase activity in human cells. Proc Natl Acad Sci USA. 1997;94:10687-10692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Zhang RG, Wang XW, Yuan JH, Xie H. Human hepatoma cell telomerase activity inhibition and cell cycle modulation by its RNA component antisense oligodeoxyribonucleotides. Acta Pharmacol Sin. 2000;21:742-746. [PubMed] |
| 27. | Zhang R, Wang X, Guo L, Xie H. Growth inhibition of BEL-7404 human hepatoma cells by expression of mutant telomerase reverse transcriptase. Int J Cancer. 2002;97:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA. 1998;95:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 313] [Article Influence: 11.6] [Reference Citation Analysis (0)] |