Published online Aug 15, 2002. doi: 10.3748/wjg.v8.i4.728
Revised: April 15, 2002
Accepted: April 23, 2002
Published online: August 15, 2002
AIM: To examine the effect of ganoderma lucidum polysaccharide (GLP) on the immune liver injury induced by BCG infection, and investigate the relationship between degrees of hepatic damage and NO production in mice.
METHODS: Immune hepatic injury was markedly induced by BCG-pretreatment (125 mg·kg-1, 2-week, iv) or by BCG-pretreatment plus lipopolysaccharide (LPS, 125 μg·kg-1, 12-hour, iv) in mice in vivo. Hepatocellular damage induced by BCG-pretreated plus inflammatory cytokines mixture (CM), which was included TNF-α, IL-1β, IFN-γ and LPS in culture medium in vitro. Administration of GLP was performed by oral or incubating with culture medium at immune stimuli simultaneity. Liver damage was determined by activity of alanine aminotransferase (ALT) in serum and in hepatocytes cultured supernatant, by liver weight changes and histopathological examination. NO production in the cultured supernatant was determined by the Griess reaction. Moreover, inducible nitric oxide synthase (iNOS) protein expression was also examinated by immunohistochemical method.
RESULTS: Immune hepatic injury was markedly induced by BCG or BCG plus inflammatory cytokines in BALB/c mice in vivo and in vitro. Under BCG-stimulated condition, augment of the liver weight and increase of the serum/supernatant ALT level were observed, as well as granuloma forming and inflammatory cells soakage were observed by microscopic analysis within liver tissues. Moreover, NO production was also increased by BCG or/and CM stimuli in the culture supernatant, and a lot of iNOS positive staining was observed in BCG-prestimulated hepatic sections. Application of GLP significantly mitigated hepatic tumefaction, decreased ALT enzyme release and NO production in serum/supernatant, improved the pathological changes of chronic and acute inflammation induced by BCG-stimuli in mice. Moreover, the immunohistochemical result showed that GLP inhibited iNOS protein expression in BCG-immune hepatic damage model.
CONCLUSION: The present study indicates that NO participates in immune liver injury induced by Mycobacterium bovis BCG infection. The mechanisms of protective roles by GLP for BCG-induced immune liver injury may be due to influence NO production in mice.
-
Citation: Zhang GL, Wang YH, Ni W, Teng HL, Lin ZB. Hepatoprotective role of
ganoderma lucidum polysaccharide against BCG-induced immune liver injury in mice. World J Gastroenterol 2002; 8(4): 728-733 - URL: https://www.wjgnet.com/1007-9327/full/v8/i4/728.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i4.728
Ganoderma lucidum polysaccharide (GLP) is an important pharmacological ingredient extracted from fruit bodies and mycelium of mushroom Ganoderma Lucidum (Fr.) Karst. It has been extensively documented that GLP can improve the damage induced by specific and nonspecific immunity responses[1,2]. In our laboratory recently studies, it was confirmed that GLP enhanced phagocytosis of intraperitoneal macrophage, inhibited the growth of implanted Sarcoma 180 and HL-60 tumor cells in vitro[3-5]. However, the regulating mechanism of GLP in the immune response remain unknown. On the other hand, hepatitis is a prevalent disease in the Chinese population. It has been recognized that the immune factors, such as virus/parasite infection, autoimmune stimuli, etc., were the dominant reasons of hepatic damage in hepatitis[6-10]. But the commonly used model of liver injury induced by chemicals does not accurately represent the clinical situation[11,12]. Therefore, It is required that development of new therapy drugs depends primarily on the availability of animal models relevant to human hepatitis or hepatocellular immune damage.
In the recently studies, Mycobacterium bovis bacillus Calmette-Guerin (BCG) infection has been proven to induce immune hepatic injury in rodent animal[13,14]. In this pathological model, the releases of hepatic endogenous cytokines, such as TNF-α, IFN-γ and IL-1β were observed in vivo[15-17]. Moreover, in our laboratory previously experiment, it has been observed that inflammatory cytokines including TNF-α and IL-1β stimulated NO production in the primary cultured rat hepatocytes in vitro[18], but the influence of GLP in this immune damage model and the exact function of NO production in the presence of inflammatory stimuli have not been elucidated yet. Therefore, the present study was performed to determine the effects of GLP on the BCG-stimulated immune liver injury in vitro and in vivo, and to investigate the possible mechanism of the influence induced by GLP in this immune response.
Following reagents were purchased from Sigma Chemical Co.: collagenase (Type IV, 340 kU·g-1), bovine insulin, and lipopolysaccharide (LPS, E. coli. 0111:B4). Other materials were obtained from the following sources: kit for determining serum and culture supernatant alanine transaminase (ALT) was from Beijing Institute of Biological Products (Beijing); Mycobacterium tuberculosis Bacille Calmette-Guerin (BCG) vaccine was from the National Vaccine and Serum Institute (Beijing); human recombinant (rh) tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interferon-gamma (IFN-γ) were from Academy of Military Medical Sciences (Beijing), and Dulbecco's modified Eagle's medium (DMEM) from Gibco BRL; Ganoderma lucidum polysaccharide (GLP) was isolated from mycelium of Ganoderma lucidum and provided by the Department of Phytochemistry, College of Pharmacy, Beijing University[5]. For using immunohistochemistry, iNOS polyclonal antibody (rabbit anti-mouse immunoglobulin) was purchased from Beijing Zhong-Shan Biotechnology co., LTD.
Male BALB/c mice weighing 18-22 g (6-8 wk old), were provided by Experimental Animal Center, Beijing University. Immune hepatic injury was induced by intravenous injection of BCG (125 mg·kg-1) for two weeks, or induced by LPS (125 μg·kg-1) for 12 h at BCG-pretreated 14 d later[13,19]. Control group mice were treated by same volume of phosphate buffered saline (PBS). After animals were BCG-pretreated 7 d, the different concentrations (25 mg·kg-1, 50 mg·kg-1, 100 mg·kg-1 and 200 mg·kg-1, respectively) of GLP were intragastric administered once at everyday within succedent one week. At immune stimulating 2 wk later, mice were killed by cervical dislocation, blood was collected and centrifuged at 3000 rpm for 5 min. Serum was obtained at the supernatant for mensuration enzyme level. Liver samples were removed rapidly for histopathological and immunohistochemical examination.
Hepatocytes were harvested from control mice or BCG-pretreated for 2 wk mice using an in situ collagenase perfusion technique[20]. After inhalation anesthesia, the abdomen of the animals was opened and shaved, the portal vein was exposed and cannulated. Then the liver was perfused at 37 °C in situ first with a calcium-free phosphate-buffered saline solution (PBS) with 6-8 mL/min velocity of flow. This perfusion was continued for 5 min, then it was switched to 0.5 g·L-1 collagenase and 10 g·L-1 bovine albumin in PBS buffer for 15 min. The liver was removed and the cells were combed gently in tissue culture medium. Hepatocytes were pelleted, washed, and separated from nonparenchymal cells by differential centrifugation at 50 × g. Viability of cells exceeded 85% as determined by trypan blue exclusion. Hapatocytes were plated onto 6-well plastic tissue-culture plates (1 × 109 cells·L-1 in each well). Medium in the control consisted of DMEM with L-arginine (0.5 mmol·L-1), insulin (1 mmol·L-1), Hepes (15 mmol·L-1), L-glutamine, penicillin, streptomycin, and 100 mL·L-1 low-endotoxin newborn calf serum. After overnight incubation, the medium was changed with a cytokines mixture (CM) containing LPS (10 mg·L-1), IL-1β (10 KU·L-1), TNF-α (500 KU·L-1) and IFN-γ (100 KU·L-1). Other experimental conditions included addition of GLP, at the different concentrations (50 mg•L-1 or 200 mg•L-1), to the CM. After primary cultures were maintained for 24 h at 37 °C in 50 mL·L-1 CO2, hepatocytes or cultured supernatants were collected for nitrite and ALT activity assays[21].
As a marker of hepatocytes necrosis, activity of alanine aminotransferase (ALT) was spectrophotometrically measured using a determinating kit in serum and culture supernatants, at 520 nM in the presence of α-ketoglutarate, aspartate, NADH and malate dehydrogenase, as described[19]. The amount of NO production in the serum and the culture supernatants were determined as its stable oxidative product, nitrite, by an automated procedure based on the Griess reaction, as previously described[20].
Livers were removed, fixed overnight in 10% buffered formalin, and paraffin-embedded. Six-micrometer sections were stained with hematoxylin-eosin for histological evaluation. Immunohistochemical staining for iNOS protein expression was carried out using rabbit polyclonal antibodies to iNOS on cryostat sections (five-micrometer). The sections were incubated with peroxidase-labeled rabbit anti-mouse immunoglobulin for 1 hour. After another wash in PBS, the sections were stained with AEC for several minutes to develop the color and washed in water. Each experiment was repeated two to three times with similar results. Three random sections of each liver were examined[19].
Data were presented with ¯x ± s, Statistical analysis was performed using ANOVA. Differences were judged to be statistically significant when the P value was less than 0.05.
Compared with the control of group, BCG-pretreatment markedly induced hepatic damage (Table 1). The augment of the liver weight and the serum ALT level were observed after BCG-administrated 2 wk in mice (P < 0.01). Furthermore, application of inflammatory lipopolysaccarides (LPS) for BCG-pretreated mice induced serum ALT activity further higher than that BCG-treated alone in mice (P < 0.05), but the liver weights were not further increased than that BCG-stimulated only groups. On the other hand, under the presence of BCG stimuli conditions, administration of CLP decreased the liver weight within the range of 50 mg·kg-1 (P < 0.05) to 200 mg·kg-1 (P < 0.01), simultaneously, serum ALT release were significantly decreased by GLP treatment in a dose-dependent manner within the similar range of concentrations (P < 0.05).
| Group | Liver weight (g) | ALT (U·L-1) |
| Control | 0.99 ± 0.16 | 22.03 ± 10.99 |
| BCG (125 mg·kg-1) | 1.79 ± 0.24b | 245.18 ± 41.03b |
| BCG (125 mg·kg-1) + LPS (125 μg·kg-1) | 1.84 ± 0.14b | 285.88 ± 23.81bc |
| BCG (125 mg·kg-1) + GLP (25 mg·kg-1) | 1.78 ± 0.20b | 236.86 ± 27.94b |
| BCG (125 mg·kg-1) + GLP (50 mg·kg-1) | 1.57 ± 0.18bc | 189.81 ± 43.99bc |
| BCG (125 mg·kg-1) + GLP (100 mg·kg-1) | 1.28 ± 0.20bd | 178.78 ± 13.16bd |
| BCG (125 mg·kg-1) + GLP (200 mg·kg-1) | 1.41 ± 0.43bc | 208.18 ± 27.93bc |
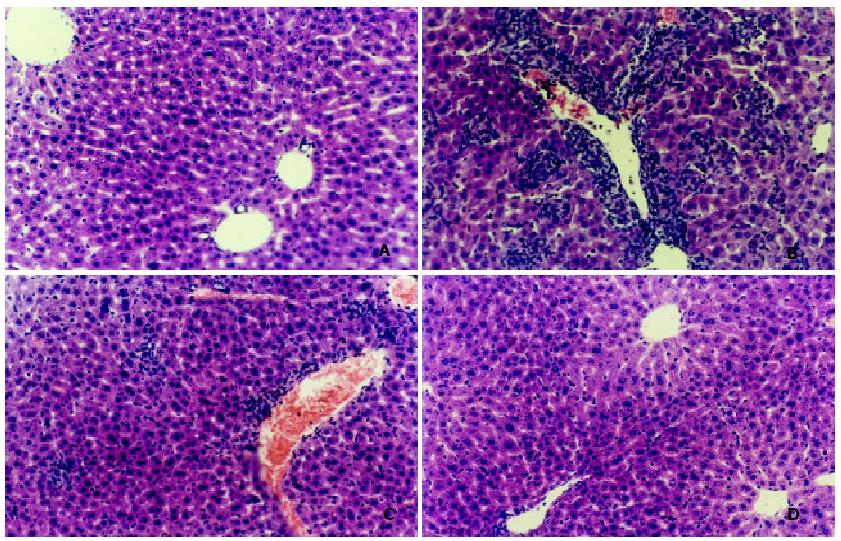
As shown in Figure 1, oppositing with the results of control group, BCG-stimulated group were observed markedly changes of liver histologic structure (Figure 1B), for example, infiltration within liver lobules by inflammatory cells, extensive hepatocytes hypertrophy, nuclear narrow, and granulation and vacuolization of the hepatocyte cytoplasm were observed in the liver section. Moreover, treatment with BCG plus LPS for mice resulted in more severe histological changes including thrombosis in the central hepatic vein and hemorrhage in the liver parenchyma (Figure 1C). Granulomas formation, a marker of chronic hepatitis fibrosis'were significantly increased by BCG-stimulated hepatic tissues (Table 2, P < 0.01). But in the presence of BCG condition, the result showh that LPS was not triggered more the granuloma forming, on the contrary, triggered more fearful hepatic tissues hemorrhage (Figure 1B , Figure 1C ).
On the other hand, the results of histological examination shown that GLP (100 mg·kg-1) alleviated hepatic damage in BCG-induced acute inflammation, such as markedly decrease of infiltration within liver lobules by inflammatory cells, nuclear narrow, etc. in the observed liver section (Figure 1D). Moreover, granulomas formation were also decreased by GLP treatment at concentration range from 100 mg·kg-1 to 200 mg·kg-1, (P < 0.01).
Effects of Ganoderma lucidum polysaccharide (GLP) on the ALT activity and NO production induced by BCG in the presence or absence of cytokines mixture (CM) in primary cultured mice hepatocytes in vitro
The result of this part of experiment shown that inflammatory cytokines increased NO production and ALT release into the supernatant in the primary cultured hepatocytes prestimulated by BCG (P < 0.01, Table 3). In the absence of cytokines condition, addition of CLP only had not influence on the activity of ALT enzyme and NO production in BCG-pretreated cultured supernatant (P > 0.05). Whereas, in the presence of inflammatory cytokines plus BCG prestimuli condition, ALT activity and NO production were markedly inhibited by application of GLP (P < 0.01).
| Group | ALT (U·L-1) | NO2- (μmol·L-1) |
| Control | 11.52 ± 1.41b | 1.41 ± 0.72a |
| BCG | 17.87 ± 3.41 | 3.52 ± 1.72 |
| BCG + GLP (50 mg·k-1) | 21.30 ± 2.87 | 3.95 ± 1.27 |
| BCG + GLP (200 mg·k-1) | 18.03 ± 2.24 | 3.24 ± 1.08 |
| BCG + Cytokines Mixture (CM) | 46.34 ± 4.17b | 13.53 ± 5.58b |
| BCG + CM + GLP (50 mg·k-1) | 23.98 ± 6.33ad | 4.11 ± 2.26d |
| BCG + CM + GLP (200 mg·k-1) | 20.61 ± 3.74d | 3.49 ± 1.38d |
CM (Cytokines mixture): IL-1β 10 KU·L-1, TNFα 500 KU·L-1, and IFNγ 100 KU·L-1 plus LPS 10 mg·L-1; Cultured hepatocytes were harvested from control group, BCG-prestimulated group in vivo, and BCG plus CM-stimulated group in vitro, respectively, in the absence or presence of GLP for 24 h; Amount of nitrite and activity of ALT in the supernatant were assayed 24 h after start of stimulation in vitro.
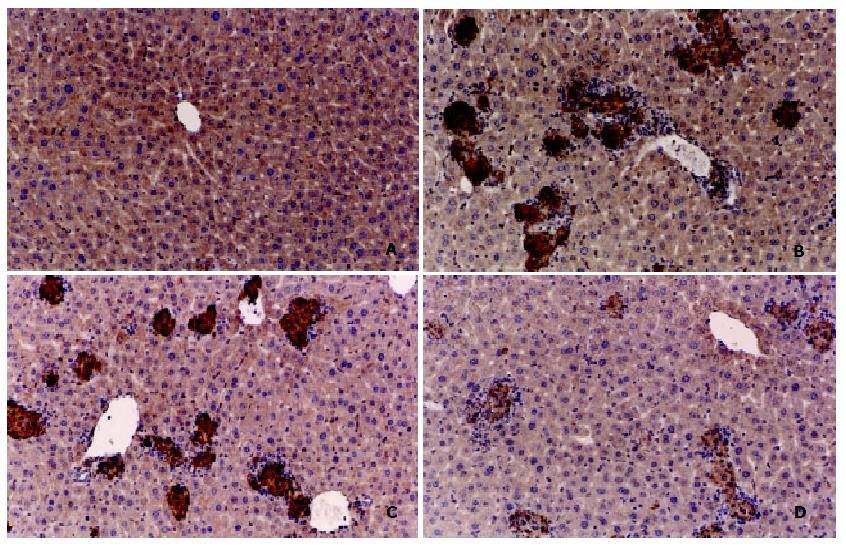
To confirm the possible mechanism about hepatoprotective role of GLP against BCG-stimulated in mice, the correlativity between iNOS expression and immune hepatic damage were investigated. As shown in the results of immunohistochemistry, compared with control group mice, there was a lot of iNOS positive brown stained agglomerate observed in BCG-stimulated hepatic section (Figure 2 A-B). But consisted with the results of granuloma forming, there were not more the iNOS expression induced by LPS in the presence of BCG stimuli condition (Figure 2C). On the contrary, treatment of GLP significantly inhibited iNOS protein expression under similary BCG-stimulated condidtion (Figure 2D).
In the present experiment, the results shown that the administration of GLP was effective against acute and chronic hepatic inflammation induced by BCG-immunostimuli in mice. Administration of GLP significantly decreased serum or supernatant ALT level in BCG-caused acute inflammatory response in vivo and in vitro. Histological changes, such as hemorrhage and necrosis in hepatic lobules, inflammatory infiltration of lymphocytes and kupffer cells around the central vein, were simultaneously improved by the treatment of GLP. These results were consistent with that GLP showed anti-inflammatory and antioxidative activities in the previous other laboratory observed results[22]. Moreover, pathohistological examination also showed that GLP decreased the granuloma formation, which is popularly considerd as the first step of fibrillar repair in the chronic inflammatory process[23-26]. This result suggested that GLP may be not only as an anti-inflammatory agent, but also may be used as an antifibrotic therapy for hepatocirrhosis.
To investigate the possible mechanisms of the hepatic protective effect of GLP in the immune-stimulated condition, we further detected NO production in primary cultured hepatocytes and iNOS protein expression in the BCG-stimulated hepatic tissues[27-30]. The results shown that GLP alone had no effect on the production of NO in the cultured hepatocytes. In the presence of BCG condition, cytokines mixture (CM) including TNF-α, IFN-γ, and LPS, significantly increased the NO production. When combined with GLP, this effect had been remarkably reversed. At the same time point, GLP also attenuated the increase of ALT activity in inflammatory cytokines-stimulated hepatocytes in vitro. It has been recognized that NO is produced by cNOS and/or iNOS in mice liver[31-37]. The results of immunohistochemistry shown that GLP effect on NO production is mainly through iNOS under immunological stimuli condition. The results of this study suggested that although the exact mechanism of action of GLP on such macrophage/lymphocyte properties of granulomas remain unknown, nevertheless, it might be related to NO production induced by cytokines[38-42]. Therefore, inhibition of NO production is partly the mechanisms of GLP protective effect on the immunological injured liver.
In summary, the present study indicates that NO participates in immune liver injury induced by Mycobacterium bovis BCG infection. Furthermore, the mechanisms of protective roles by GLP for BCG-induced immune liver injury in mice may be due to influence NO production. However, further study is needed to understand the exact mechanisms of the antihepatotoxic activity and the free radical scavenging activity of GLP. The clinical applicability of GLP remains to be established.
Edited by Pang LH
Presented at the third international symposium on hepatology, Hangzhou, China, 18-23 October, 2001
| 1. | Bao X, Liu C, Fang J, Li X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr Res. 2001;332:67-74. [PubMed] |
| 2. | Cheung WM, Hui WS, Chu PW, Chiu SW, Ip NY. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000;486:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Ma L, Lin ZB. Effects of Ganoderma polysaccharides on IL-2 production by mouse splenocytes in vitro. J Beij Med Univ. 1991;23:412-417. |
| 4. | Lei LS, Lin ZB. Effect of Ganoderma polysaccharides on T cell subpopulations and production of interleukin 2 in mixed lymphocyte response. Yaoxue Xuebao. 1992;27:331-335. [PubMed] |
| 5. | Zhang QH, Lin ZB. The antitumor activity of Ganoderma lucidum (Curt.: Fr.) P. Karst. (Ling Zhi) (Aphyllophoromy cetideae) polysaccharides is related to tumor nerosis factor-a and interferon-γ. Inter J Med Mushrooms. 1999;1:207-215. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ouyang EC, Wu CH, Walton C, Promrat K, Wu GY. Transplantation of human hepatocytes into tolerized genetically immunocompetent rats. World J Gastroenterol. 2001;7:324-330. [PubMed] |
| 7. | Guo SP, Wang WL, Zhai YQ, Zhao YL. Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340-344. [PubMed] |
| 8. | You J, Zhuang L, Tang BZ, Yang WB, Ding SY, Li W, Wu RX, Zhang HL, Zhang YM, Yan SM. A randomized controlled clinical trial on the treatment of Thymosin a1 versus interferon-alpha in patients with hepatitis B. World J Gastroenterol. 2001;7:411-414. [PubMed] |
| 9. | Li XW, Ding YQ, Cai JJ, Yang SQ, An LB, Qiao DF. Studies on mechanism of Sialy Lewis-X antigen in liver metastases of human colorectal carcinoma. World J Gastroenterol. 2001;7:425-430. [PubMed] |
| 10. | Liu BH, Chen HS, Zhou JH, Xiao N. Effects of endotoxin on endothelin receptor in hepatic and intestinal tissues after endotoxemia in rats. World J Gastroenterol. 2000;6:298-300. [PubMed] |
| 11. | Cheng JL, Liu BL, Zhang Y, Tong WB, Yan Z, Feng BF. Hepatitis C virus in human B lymphocytes transformed by Epstein-Barr virus in vitro by in situ reverse transcriptase-polymerase chain reaction. World J Gastroenterol. 2001;7:370-375. [PubMed] |
| 12. | Zhuang L, You J, Tang BZ, Ding SY, Yan KH, Peng D, Zhang YM, Zhang L. Preliminary results of Thymosin-a1 versus interferon-alpha-treatment in patients with HBeAg negative and serum HBV DNA positive chronic hepatitis B. World J Gastroenterol. 2001;7:407-410. [PubMed] |
| 13. | Carpenter E, Fray L, Gormley E. Antigen-specific lymphocytes enhance nitric oxide production in Mycobacterium bovis BCG-infected bovine macrophages. Immunol Cell Biol. 1998;76:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Wang GS, Liu GT. Role of nitric oxide in immunological liver damage in mice. Biochem Pharmacol. 1995;49:1277-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Bai XY, Jia XH, Cheng LZ, Gu YD. Influence of IFN alpha-2b and BCG on the release of TNF and IL-1 by Kupffer cells in rats with hepatoma. World J Gastroenterol. 2001;7:419-421. [PubMed] |
| 16. | Erb KJ, Kirman J, Delahunt B, Chen W, Le Gros G. IL-4, IL-5 and IL-10 are not required for the control of M. bovis-BCG infection in mice. Immunol Cell Biol. 1998;76:41-46. [PubMed] |
| 17. | Ugaz EM, Pinheiro SR, Guerra JL, Palermo-Neto J. Effects of prenatal diazepam treatment on Mycobacterium bovis-induced infection in hamsters. Immunopharmacology. 1999;41:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Zhang GL, Lin ZB. Effects of cytokines on the endotoxin stimulated nitric oxide production in the primary cultured rat hepatocytes. Beijing Yike Daxue Xuebao. 1998;30:180-182. |
| 19. | Zhang G, Lin Z, Zhang B. [Effects of selective induceble nitric oxide synthase inhibitor on immunological hepatic injury in rat]. Zhonghua Yixue Zazhi. 1998;78:540-543. [PubMed] |
| 20. | Zhang GL, Lin ZB. Dinoprostone potentiates cytokines and lipopolysaccharides inducing nitric oxide production in cultured rat hepatocytes. Zhongguo Yaoli Xuebao. 1999;20:262-266. [PubMed] |
| 21. | Zhang GL, Wang YH, Teng HL, Lin ZB. Effects of aminoguanidine on nitric oxide production induced by inflammatory cytokines and endotoxin in cultured rat hepatocytes. World J Gastroenterol. 2001;7:331-334. [PubMed] |
| 22. | Lee JM, Kwon H, Jeong H, Lee JW, Lee SY, Baek SJ, Surh YJ. Inhibition of lipid peroxidation and oxidative DNA damage by Ganoderma lucidum. Phytother Res. 2001;15:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363-369. [PubMed] |
| 24. | Huang YQ, Xiao SD, Mo JZ, Zhang DZ. Effects of nitric oxide synthesis inhibitor in long term treatment on hyperdynamic circulatory state in cirrhotic rats. World J Gastroenterol. 2000;6:31. |
| 25. | Feng ZJ, Feng LY, Sun ZM, Song M, Yao XX. Expression of nitric oxide synthase protein and gene in the splanchnic organs of liver cirrhosis and portal hypertensive rats. World J Gastroenterol. 2000;6:33. |
| 26. | Vernia S, Beaune P, Coloma J, López-García MP. Differential sensitivity of rat hepatocyte CYP isoforms to self-generated nitric oxide. FEBS Lett. 2001;488:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Wang JH, Redmond HP, Wu QD, Bouchier-Hayes D. Nitric oxide mediates hepatocyte injury. Am J Physiol. 1998;275:G1117-G1126. [PubMed] |
| 28. | Alexander B. The role of nitric oxide in hepatic metabolism. Nutrition. 1998;14:376-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Kaibori M, Sakitani K, Oda M, Kamiyama Y, Masu Y, Nishizawa M, Ito S, Okumura T. Immunosuppressant FK506 inhibits inducible nitric oxide synthase gene expression at a step of NF-kappaB activation in rat hepatocytes. J Hepatol. 1999;30:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Moriyama A, Tabaru A, Unoki H, Abe S, Masumoto A, Otsuki M. Plasma nitrite/nitrate concentrations as a tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2000;296:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Hara H, Mitani N, Adachi T. Inhibitory effect of nitric oxide on the induction of cytochrome P450 3A4 mRNA by 1, 25-dihydroxyvitamin D3 in Caco-2 cells. Free Radic Res. 2000;33:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Jun Y, Fei G, Ebert MP, Malfertheiner P. Expression of inducible nitric oxide synthase in human gastric cancer. World J Gastroenterol. 1999;5:430-431. [PubMed] |
| 34. | Ji XL, Shen MS, Yin T. Liver inflammatory pseudotumor or parasitic granuloma? World J Gastroenterol. 2000;6:458-460. [PubMed] |
| 35. | Heneka MT, Löschmann PA, Gleichmann M, Weller M, Schulz JB, Wüllner U, Klockgether T. Induction of nitric oxide synthase and nitric oxide-mediated apoptosis in neuronal PC12 cells after stimulation with tumor necrosis factor-alpha/lipopolysaccharide. J Neurochem. 1998;71:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Liu SH, Tzeng HP, Kuo ML, Lin-Shiau SY. Inhibition of inducible nitric oxide synthase by beta-lapachone in rat alveolar macrophages and aorta. Br J Pharmacol. 1999;126:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Vos TA, Gouw AS, Klok PA, Havinga R, van Goor H, Huitema S, Roelofsen H, Kuipers F, Jansen PL, Moshage H. Differential effects of nitric oxide synthase inhibitors on endotoxin-induced liver damage in rats. Gastroenterology. 1997;113:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Tzeng E, Billiar TR, Williams DL, Li J, Lizonova A, Kovesdi I, Kim YM. Adenovirus-mediated inducible nitric oxide synthase gene transfer inhibits hepatocyte apoptosis. Surgery. 1998;124:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Nomura T, Ohtsuki M, Matsui S, Sumi-Ichinose C, Nomura H, Hagino Y. Nitric oxide donor NOR 3 inhibits ketogenesis from oleate in isolated rat hepatocytes by a cyclic GMP-independent mechanism. Pharmacol Toxicol. 1998;82:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Imagawa J, Yellon DM, Baxter GF. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. Br J Pharmacol. 1999;126:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Tunçtan B, Uludag O, Altug S, Abacioglu N. Effects of nitric oxide synthase inhibition in lipopolysaccharide-induced sepsis in mice. Pharmacol Res. 1998;38:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Ohmori H, Egusa H, Ueura N, Matsumoto Y, Kanayama N, Hikida M. Selective augmenting effects of nitric oxide on antigen-specific IgE response in mice. Immunopharmacology. 2000;46:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |