Published online Apr 15, 2002. doi: 10.3748/wjg.v8.i2.237
Revised: October 22, 2001
Accepted: October 29, 2001
Published online: April 15, 2002
AIM: The survival time of patients with hepatocellular carcinoma (HCC) after resection is hard to predict. Both residual liver function and tumor extension factors should be considered. A new scoring system has recently been proposed by the Cancer of the Liver Italian Program (CLIP). CLIP score was confirmed to be one of the best ways to stage patients with HCC. To our knowledge, however, the literature concerning the correlation between CLIP score and prognosis for patients with HCC after resection was not published. The aim of this study is to evaluate the recurrence and prognostic value of CLIP score for the patients with HCC after resection.
METHODS: A retrospective survey was carried out in 174 patients undergoing resection of HCC from January 1986 to June 1998. Six patients who died in the hospital after operation and 11 patients with the recurrence of the disease were excluded at 1 mo after hepatectomy. By the end of June 2001, 4 patients were lost and 153 patients with curative resection have been followed up for at least three years. Among 153 patients, 115 developed intrahepatic recurrence and 10 developed extrahepatic recurrence, whereas the other 28 remained free of recurrence. Recurrences were classified into early (≤ 3 year) and late (> 3 year) recurrence. The CLIP score included the parameters involved in the Child-Pugh stage (0-2), plus macroscopic tumor morphology (0-2), AFP levels (0-1), and the presence or absence of portal thrombosis (0-1). By contrast, portal vein thrombosis was defined as the presence of tumor emboli within vascular channel analyzed by microscopic examination in this study. Risk factors for recurrence and prognostic factors for survival in each group were analyzed by the χ² test, the Kaplan-Meier estimation and the COX proportional hazards model respectively.
RESULTS: The 1-, 3-, 5-, 7-, and 10-year disease-free survival rates after curative resection of HCC were 57.2%、28.3%、23.5%、18.8% and 17.8%, respectively. Median survival time was 28, 16, 10, 4, and 5 mo for CLIP score 0, 1, 2, 3, and 4 to 5, respectively. Early and late recurrence developed in 109 patients and 16 patients respectively. By the χ² test, tumor size, microsatellite, venous invasion, tumor type (uninodular, multinodular, massive), tumor extension (≤ or > 50% of liver parenchyma replaced by tumor), TNM stage, CLIP score, and resection margin were the risk factors for early recurrence, whereas CLIP score and Child-Pugh stage were significant risk factors for late recurrence. In univariate survival analysis, Child-Pugh stages, resection margin, tumor size, microsatellite, venous invasion, tumor type, tumor extension, TNM stages, and CLIP score were associated with prognosis. The multivariate analysis by COX proportional hazards model showed that the independent predictive factors of survival were resection margins and TNM stages.
CONCLUSION: CLIP score has displayed a unique superiority in predicting the tumor early and late recurrence and prognosis in the patients with HCC after resection.
- Citation: Zhao WH, Ma ZM, Zhou XR, Feng YZ, Fang BS. Prediction of recurrence and prognosis in patients with hepatocellular carcinoma after resection by use of CLIP score. World J Gastroenterol 2002; 8(2): 237-242
- URL: https://www.wjgnet.com/1007-9327/full/v8/i2/237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i2.237
Hepatocellular carcinoma (HCC) is one of the major causes of death in the world[1-4]. In China, HCC ranked second of cancer mortality since 1990s[5]. Within the past decade, as a result of advances in the diagnosis and disease management of HCC, significant improvement of overall and disease-free survival rates after resection of HCC have been achieved[6-9]. However, even when curative resection is performed, a considerable number of patients develop intrahepatic and/or extrahepatic recurrence postoperatively[10-16]. The recurrence and prognostic assessment of patients with HCC after resection is an important clinical issue[13,14,17-21].
After Okuda made a suggestion of clinical stage system[22], UICC introduced the tumor node metastasis (TNM) stage that was widely used in China for predicting the prognosis of HCC[17,18,23]. TNM stage took into account of the tumor extension alone and it did not deal with liver function. HCC mainly arose in patients already affected with chronic hepatitis or cirrhosis[12,24]. The prevalence of hepatitis B surface antigen (HbsAg) and antibody to HCV in HCC patients were reported to be 63.2% and 11.2% respectively in China[25], and coexisting cirrhosis occurred in 88.8% (888/1000 patients) for patients with small HCC[14]. The liver damage in patients with HCC after resection is also a risk factor for recurrence and prognosis[26-32]. Hence, a new stage accounting for both liver function and tumor extension is necessary to predict HCC prognosis.
In 1998, by a retrospective evaluation of 435 Italian patients, the Cancer of the Liver Italian Program (CLIP) had proposed a new prognostic system that included four parameters involved in the Child-Pugh stag, plus macroscopic tumor morphology, alpha-fetoprotein (AFP) levels, and the presence or absent portal thrombosis and confirmed that the CLIP score, compared with the Okuda staging system, gave more accurate prognostic information, was statistically more efficient, and had a greater survival predictive power[33,34]. After that, Farinati et al[35] pointed that the CLIP score was able to predict survival better than TNM staging system, especially in the subgroup of patients undergoing chemoembolization. However, the number of patients with HCC after resection in these two studies was small and limited. Correlation between CLIP score and prognosis of patients with HCC after resection was not reported. The aim of this study is to validate the prognostic value of CLIP score in the patients with HCC by means of disease-free survival rate and time to recurrence for patients enrolled between 1986 and 1998 in our department.
From January 1986 to June 1998, 174 patients underwent resection of HCC at our department. The mean age was 49.8 (range, 9-75) years old and the ratio of male to female was 156 to 18. Six patients who died in the hospital after operation and 11 patients were excluded because of the disease detected in the remnant liver by a routine ultrasound or CT scan or arteriography performed at 1 mo after hepatectomy, which was considered residual disease. By the end of June 2001, 4 patients were lost and 153 patients with curative resection have been followed up for at least three years. Among 153 patients, 115 developed intrahepatic recurrence and 10 developed extrahepatic recurrence, whereas the other 28 remained recurrence free after a mean follow up period of 60 mo. Figure 1 showed the patients distribution of the time to recurrences or death after operation. There were two peaks at 6 mo and 15 mo among those with early recurrence. Our data indicated that 3-year' survival after hepatectomy was a distinctive point of early from late recurrence when compared with other time intervals such as 1 and 2 years.
The CLIP score system included Child-Pugh stage[36], tumor morphology and extension, AFP levels, and the presence or absence of portal thrombosis (Table 1).
| Variable | Scores | ||
| 0 | 1 | 2 | |
| Child-Pugh stage | A | B | C |
| Tumor morphology | Uninodular and extension ≤ 50% | Multinodular and extension ≤ 50% | Massive or extension > 50% |
| AFP (ng/Dl) | < 400 | ≥ 400 | |
| Portal vein thrombosis | No | Yes | |
The scores was calculated by addition of each individual value of the four items[34]. What was the difference from the CLIP data, that portal vein thrombosis was defined as the presence of tumor emboli within vascular channel analyzed by microscopic examination in this study.
Disease-free survival rate was defined as the time relapsed from the date of image diagnosis and either the date of death or the date of the latest follow-up visit, with final evaluation at June 30, 2000. Recurrences were classified into early (≤ 3 year) and late recurrence (> 3 year) after the date of hepatectomy. Patients with early or late recurrences were compared respectively with patients who did not develop any recurrent disease during the follow-up periods to identify risk factors.
Comparisons between groups were performed by the χ² test (Pearson's methods or Fisher's exact test). Univariate survival curves were evaluated using the Kaplain-Meier method and compared by using the Long-rang test. The COX proportional hazards model was used to determine the significance of each significant univariate variable in multivariate analysis of prognostic factors. All analyses were performed using SPSS 8.0 for windows software. All P values of less than 0.05 in two-tailed were considered statistically significant.
The 1-, 3-, 5-, 7-, and 10-year cumulative disease-free survival rates were 57.2%, 28.3%, 23.5%, 18.8% and 17.8% respectively after hepatectomy. Among the 125 patients with recurrence, 109 had early recurrences and 16 had late recurrences. 28 patients were recurrence free within follow-up period.
The characteristics of the host-and treatment-related factors were showed in Table 2. Among of six host-related factors, age, gander, hepatitis B surface antigen status, tumor family history and cirrhosis were associated with neither early nor late recurrence.
| Characteristics | Early recurrence (n = 109) | Late recurrence (n = 16) | No recurrence (n = 28) |
| Age | 50.7 ± 9.8 | 49.2 ± 8.5 | 48.3 ± 10.2 |
| Gander (M/F) | 100/9 | 13/3 | 24/4 |
| HbsAg | |||
| positive | 78 | 12 | 18 |
| negative | 31 | 4 | 10 |
| Tumor family history | |||
| Yes | 16 | 5 | 4 |
| No | 91 | 11 | 24 |
| Unknown | 2 | 0 | 0 |
| Cirrhosis | |||
| Yes | 82 | 12 | 18 |
| No | 27 | 4 | 10 |
| Child-Pugh | |||
| A | 91 | 10 | 26 |
| B | 31 | 6 | 2 |
| C | 1 | 0 | 0 |
| Preoperative TAE | |||
| Yes | 18 | 0 | 4 |
| No | 97 | 16 | 24 |
| splenectomy | |||
| Yes | 11 | 1 | 4 |
| No | 98 | 15 | 24 |
| Resection margin (cm) | |||
| < 1 | 67 | 4 | 4 |
| ≥ 1 | 42 | 12 | 24 |
| Postoperative chemotherapy | |||
| Yes | 58 | 9 | 13 |
| No | 51 | 7 | 15 |
Child-Pugh stage had not correlation with early recurrence, whereas it had significant correlation with late recurrence [Child stage B: 37.5% (6/16) in late recurrence versus 7.1% (2/28) in no-recurrence, P = 0.019]. The four treatment-related factors, preoperative transcatheter arterial embolization (TAE), postoperative chemotherapy, with or without splenectomy, were associated with neither early nor late recurrence. The proportion of resection margin less than one centimeter in early recurrence was significant higher than that in no-recurrence (P = 0.000), but had not correlated with late recurrence. Eight tumor-related factors except serum AFP levels were associated with early recurrence, namely tumor size > 5 cm (P = 0.001), presence of microsatellite (P = 0.000), venous invasion (P = 0.001), tumor type (P = 0.024), tumor extension (P = 0.013), advanced TNM stages (P = 0.000), and CLIP scores (P = 0.005). In contrast, only CLIP score has significant correlation with late recurrence [CLIP score 0-1: 92.9% (26/28) in no-recurrence versus 56.2% (9/16) in late recurrence, P = 0.006] (Table 3).
| Characteristics | Early recurrence (n = 109) | Late recurrence (n = 16) | No recurrence (n = 28) |
| Serum AFP ( μg•L⁻¹) | |||
| < 400 | 66 | 8 | 14 |
| ≥ 400 | 43 | 8 | 14 |
| Tumor size/cm | |||
| ≤ 5 | 43* | 7 | 21 |
| > 5 | 66 | 9 | 7 |
| Microsatellite | |||
| Absent | 69 | 14 | 28 |
| present | 40 | 2 | 0 |
| Venous invasion | |||
| Absent | 78 | 15 | 28 |
| present | 31 | 1 | 0 |
| Tumor type | |||
| Uninodular | 78 | 14 | 27 |
| Multinodular | 17 | 2 | 1 |
| Massive | 14 | 0 | 0 |
| Tumor extension | |||
| ≤ 50% | 90 | 15 | 28 |
| > 50% | 19 | 1 | 0 |
| UICC TNM stage | |||
| I | 1 | 1 | 9 |
| II | 36 | 11 | 18 |
| III | 51 | 12 | 1 |
| IV | 21 | 2 | 0 |
| CLIP scores | |||
| 0 | 21 | 6 | 11 |
| 1 | 38 | 3 | 15 |
| 2 | 25 | 6 | 1 |
| 3 | 4 | 0 | 1 |
| 4-5 | 11 | 1 | 0 |
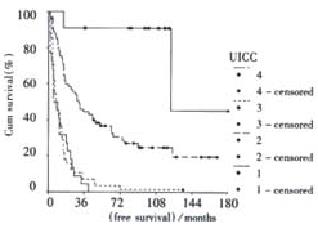
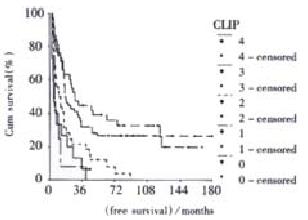
Univariate survival curve analysis showed that Child stage (P = 0.0358), resection margin (P = 0.0000), tumor size (P = 0.0000), presence of microsatellite (P = 0.0000), venous invasion (P = 0.0000), tumor type (P = 0.0000), tumor extension (P = 0.0000), TNM stage (P = 0.0000), and CLIP score (P = 0.0000) were associated with prognosis. The multivariate analysis by COX proportional hazards model revealed that the independent prognostic factors for survival were resection margin (P = 0.0000) and TNM stage (P = 0.0035). Figure 2 and Figure 3 demonstrated cumulative disease free survival curves of TNM stage and CLIP score respectively. Table 3 showed 1-, 3-, and 5-year disease free survival rates and median survival time of TNM stage and CLIP score.
Recurrences of HCC in the remnant after surgical resection could originate from either intrahepatic metastasis from the primary tumor or multicentric occurrence[19,32,37,38]. Some authors have been found that recurrence was mainly associated with adverse tumor factors such as tumor size, vascular invasion, and microsatellite[10-14,17,18]. Others demonstrated a liver function status[26-32,38]. Poon et al[19] showed that tumor factors were linked to early recurrence, whereas the nontumorous liver status was linked to late recurrence. Thus, a good stage system should include both tumor-related factors and host-related factor. In 1998, a retrospective analysis of 435 cases of HCC diagnosed at 16 Italian institutions was performed by CLIP[33]. Four independent predictive factors of survival were Child-Pugh stage, tumor morphology, AFP, and portal vein thrombosis by multivariate analysis. Adding each individual value for the four items, the CLIP score was produced. The median survival time for CLIP score 0, 1, 2, 3, 4, and 5 to 6 were 42.5, 32.0, 16.5, 4.5, 2.5, and 1.0 mo respectively. Compared with Okuda stage I (32.5 mo), II (12.0 mo), and III (1.5 mo), the CLIP score has a higher number of categories and a greater discriminating ability, and gives more precise information[33,34]. After that, 154 patients with histologically ascertained HCC and cirrhosis were recruited by Farinati et al[35] and found that the median survival rate in patients with CLIP stages 0, 1, 2, 3, 4, and 5 to 6 were 31, 27, 13, 8, 2, and 2 mo respectively. The TNM staging was much less effective in distinguishing patients with respect to median survival (TNM stage I, 34 mo; stage II, 25 mo; stage III, 20 mo; and stage IV, 14 mo) and TNM was not significantly correlated with survival rate in patients undergoing a single treatment (TACE). In conclusion, Farinati et al[35] confirmed that the CLIP score was currently the best way to stage patients with HCC, it could accurately identify patients with different prognosis, particularly in the early phases (CLIP0-3) of HCC. However, in those data, the major treatment of the HCC were not surgical resection, 12 of 435 patients in the CLIP group[33] and 17 of 154 patients in Farinati group[35] were surgical treatment. The CLIP score was based on prognostic parameters of unresectable patients. This study introduced the CLIP score into the patients with HCC after resection and studied its prognostic value. The results suggested that CLIP score were significantly associated with early and late recurrence, but TNM stage was only associated with early recurrence. It meant that the risk of early recurrence was dependent on the primary tumor factors, but the late recurrence was dependent on the underlying liver status. The CLIP score has a unique superiority for predicting recurrence, because of that accounting for both liver function and tumor extension. The survival analysis showed that the CLIP score was associated with prognosis in the univariate survival and did not associated with that in the multivariate survival. Otherwise, the TNM stage was associated with prognosis in the both univariated and multivariated survival analysis. We had reported before[18] that the TNM stage was inefficient for multicentric origin and Long-term survival patients because the TNM stage depended only on tumor characteristics, but not on patient factors[32]. In addition, CLIP score also solved out a problem of TNM stage, that is HCC with two nodes located in both lobes of the liver may be a multicentric origin and the patient after complete resection will have a good prognosis, which was identified stage IV in TNM stage. Recently, Ueno et al[39] investigated the value of CLIP score in 662 Japanese patients with HCC at 4 Japanese institutions from 1990 and 1998. Compared with the Okuda and AJCC staging systems, the CLIP score revealed a class of patients with an impressively more favorable prognosis and another class with a relatively shorter life expectancy. Moreover, the likelihood ratio test showed that the CLIP score had additional homogeneity of survival within each score above that of the Okuda stage or the AJCC stage. This was true for 3 subgroups of patients who received surgery, TAE, and percutaneous ethanol injections.
The patients with portal vein tumor thrombosis (PVTT), which was one of the most important predictive factor identified with the multivariated analysis[10-12,14,17,18,40-42] showed higher recurrence rate and a poor prognosis, as has been found previously. However, unlike the resectable cases, the PVTT had been a relatively rare condition prevalently in unresectable patients[43]. With the development of image technology, the CT and Doppler ultrasound characteristics of benign and malignant PVTT have been described[43,44]. But the PVTT detected by image examination were either located in the main and/or the first portal branch or large thrombosis[43,44]. For the resectable HCC, macroscopic tumor thrombus rate was 4.9%[14]. Cong et al[45] reported the rate of microscopic vascular embolus was 28% in 93 hepatectomies of < 3 cm small HCC. In the 336 patients with HCC reported by Sithinamsuwan et al[40], the PVTT was 50%. Liver Cancer Study Group of Japan[11] reported that image TVPP positive rate was 23%, whereas microscopic PVTT rate was 72.8%. Thus, whether the portal thrombosis was benign or malignant, and PVTT was defined by image or pathology, those all would affect patient's prognosis, and result in the difference of scores of CLIP stage. Under similar circumstances, it will effect predictive capacity of CLIP score.
AFP level is the most effective marker for HCC diagnosis and recurrent surveillance[11,46-48]. The prognostic significance of AFP level is still a controversial issue[11,12,14,17,18,33,40,46,48]. The large series data (Liver Cancer Study Group of Japan in 1995, 9099 patients[11], Zhang in 2000, 1725 patients[17]) showed that AFP level was associated with prognosis, and the higher AFP was, the poorer patient's prognosis was. To our knowledge, no study has been reported that AFP level has correlated with prognosis in multivariate analysis in China[17,18,49,50], and the literature also did not show any relationship between AFP level and prognosis. Rabe et al[12] reported that while AFP positivity itself was not associated with lower survival, AFP-level greater than 100 μg•L⁻¹ was associated with a median survival of 3 mo, and lower AFP-level was associated with a survival of 12.5 mo (P < 0.01). Hanazaki et al[51] reported that AFP > or = 1000 μg•L⁻¹ and the presence of vascular invasion were independent unfavorable prognostic factors affecting overall survival and that AFP > or = 1000 μg•L⁻¹ was an independently significant factor of poor disease-free survival. Thus, the both all were inefficient and need to be considered that the critical value of AFP was more than 400 μg•L⁻¹ and the prognostic weight of preoperative variable AFP level was defined zero or one score in CLIP, which was the same as present or absent portal vein thrombosis.
Tumor morphology, including the microsatellite, tumor size, uni-or multinodular and tumor extension, can be regarded as indices of tumor burden and was defined as prognostic factors previously for patients with HCC[9,18,33-35,41,49]. Recently, the literature regarding the correlation of remnant liver function status and prognosis after operation, increased gradually. 246 patients with curative resection of HCC were observed. Intrahepatic recurrences were classified into early (≤ 1 year) and late (> 1 year) recurrences. By multivariate analysis, preoperative tumor rupture and venous invasion were independent risk factors for early recurrence, whereas cirrhosis was the only significant risk factor for late recurrence. By comparing histological features of resected recurrent and primary tumors, 8 of 9 resected early recurrent tumors (89%) were classified as intrahepatic metastases, whereas all 6 resected late recurrent tumors (100%) were multicentric occurrences[19]. For resectable cases of HCC, the most patients have a good liver function in preoperation, whereas the late recurrence or death only associated with Child stage in this study, which suggest that liver function damage is the one of reasons which result in late death (no-tumor factors)beside early operative mortality or easy to recurrence. Ariizumi et al[52] showed that multicentric HCC tended to grow in more damaged segments of the liver. Thus, influence of tumor-related factors to patients who survived more than three years with no recurrence decreased gradually, meanwhile the influence of host-related factors, including liver functional status and multicentric in origin increased gradually[18,32]. Consequently, a question occurs: why HCC only found some patients but not all the answer only is that the liver background with or without HCC is different from each other. In other word, some livers have a predisposition to cancer. Donato et al[53] have studied risk factors and clinical significance of high liver cell proliferative activity in 208 well-compensated cirrhotic patients with immunostaining for proliferating cell nuclear antigen (PCNA). The overall PCNA labeling index (LI) ranged from 0.1% to 12.5% (mean, 2.1%), being significantly higher in the 50 patients who developed HCC during 88 ± 42 mo of follow-up than in the 158 patients who remained cancer-free (3.6% ± 2.4% vs 1.6% ± 1.5%; P < 0.0001). In conclusion, development of HCC in patients with compensated cirrhosis seems to be reliably predicted by liver cell proliferation status. Sangiovanni et al[54] assessed whether hepatocyte proliferation is an independent risk factor for HCC when considered together with clinical and demographic characteristics. Total 97 consecutive patients with a histological diagnosis of cirrhosis and preserved liver tissue function were retrospectively evaluated, Hepatocyte proliferation was evaluated by flow-cytometric analysis in liver samples collected at the time of histological diagnosis of cirrhosis. The results showed that S-phase fraction of 1.8% or higher significantly correlated with the development of HCC. To study the liver status of patients with HCC have important role in clarification of histogenesis of HCC.
In summary, CLIP score, because of giving consideration to both tumor and patients, has displayed a unique superiority in predicting the tumor recurrence and prognosis in patients with HCC.
Edited by Zhang JZ
| 1. | Tang ZY. Advances in clinical research of hapatocellular carcinoma in China. World J Gastroenterol. 1998;4:4-7. [Cited in This Article: ] |
| 2. | Yip D, Findlay M, Boyer M, Tattersall MH. Hepatocellular carcinoma in central Sydney: A 10-year review of patients seen in a medical oncology department. World J Gastroenterol. 1999;5:483-487. [PubMed] [Cited in This Article: ] |
| 3. | Schmid R. Prospect of gastroenterology and hepatology in the next century. World J Gastroenterol. 1999;5:185-190. [PubMed] [Cited in This Article: ] |
| 4. | Roberts LR, LaRusso NF. Potential roles of tumor suppressor genes and microsatellite instability in hepatocellular carcinogenesis in southern African blacks. World J Gastroenterol. 2000;6:37-41. [PubMed] [Cited in This Article: ] |
| 5. | Zhang S, Li L, Lu F. [Mortality of primary liver cancer in China from 1990 through 1992]. Zhonghua Zhongliu Zazhi. 1999;21:245-249. [PubMed] [Cited in This Article: ] |
| 6. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: A prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 454] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 7. | Parks RW, Garden OJ. Liver resection for cancer. World J Gastroenterol. 2001;7:766-771. [PubMed] [Cited in This Article: ] |
| 8. | Wu MC, Shen F. Progress in research of liver surgery in China. World J Gastroenterol. 2000;6:773-776. [PubMed] [Cited in This Article: ] |
| 9. | Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. World J Gastroenterol. 1998;4:4-7. [Cited in This Article: ] |
| 10. | Paquet KJ, Lazar A, Heine WP, Jachmann-Jahn V. [Small unilocular hepatocellular carcinoma (0 & lt; 5 cm) in patients with liver cirrhosis. Early diagnosis, surgical indications, resection and prognosis]. Zentralbl Chir. 2000;125:629-636. [PubMed] [Cited in This Article: ] |
| 11. | Liver cancer study group of Japan. Survey and follow-up study of primary liver cancer in Japan-Report 11. Liver. 1995;36:208-217. [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208-215. [PubMed] [Cited in This Article: ] |
| 13. | Ono T, Yamanoi A, Nazmy El Assal O, Kohno H, Nagasue N. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: metaanalysis of three randomized controlled trials. Cancer. 2001;91:2378-2385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 14. | Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL, Wu ZQ, Fan J, Qin LX, Zheng BH. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 15. | Wu MC. Clinical research advances in primary liver cancer. World J Gastroenterol. 1998;4:471-474. [PubMed] [Cited in This Article: ] |
| 16. | Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61-65. [PubMed] [Cited in This Article: ] |
| 17. | Zhang Z, Wu M, Shen F. [Significance of TNM clasification in prognostic evaluation of hepatocelluar carcinoma following surgical resection]. Zhonghua Zhongliu Zazhi. 1999;21:293-295. [PubMed] [Cited in This Article: ] |
| 18. | Zhao WH, Feng YZ, Ma ZM, Zhou XR. Study on prognostic factors for hepatocellular carcinoma after resection by Cox proportional hazard. Zhonghua Putong Waike Zazhi. 2001;16:472-474. [Cited in This Article: ] |
| 19. | Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 20. | Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91:771-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, Tanikawa M, Sone Y, Hisanaga Y. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer. 2001;91:957-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 22. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 23. | Yan LN, Zeng Y, Wen TF, Zhou JC, Lu SC, Li P, Chen XL. The feasibility of a new clinical staging for primary liver cancer. Zhonghua Putong Waike Zazhi. 2001;16:455-456. [Cited in This Article: ] |
| 24. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] [Cited in This Article: ] |
| 25. | Zhang JY, Dai M, Wang X, Lu WQ, Li DS, Zhang MX, Wang KJ, Dai LP, Han SG, Zhou YF. A case-control study of hepatitis B and C virus infection as risk factors for hepatocellular carcinoma in Henan, China. Int J Epidemiol. 1998;27:574-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Takata M, Yamanaka N, Tanaka T, Yamanaka J, Maeda S, Okamoto E, Yasojima H, Uematsu K, Watanabe H, Uragari Y. What patients can survive disease free after complete resection for hepatocellular carcinoma: A multivariate analysis. Jpn J Clin Oncol. 2000;30:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. 1999;86:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 28. | Hanazaki K, Wakabayashi M, Sodeyama H, Kajikawa S, Amano J. Hepatic function immediately after hepatectomy as a significant risk factor for early recurrence in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:3201-3207. [PubMed] [Cited in This Article: ] |
| 29. | Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu CL, Wong J. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: A study of a prospective cohort. J Clin Oncol. 2001;19:3037-3044. [PubMed] [Cited in This Article: ] |
| 30. | Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18:1094-1101. [PubMed] [Cited in This Article: ] |
| 31. | Ko S, Nakajima Y, Kanehiro H, Hisanaga M, Aomatsu Y, Kin T, Yagura K, Ohyama T, Nishio K, Ohashi K. Significant influence of accompanying chronic hepatitis status on recurrence of hepatocellular carcinoma after hepatectomy. Result of multivariate analysis. Ann Surg. 1996;224:591-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Yamasaki S, Kosuge T, Yamamoto J, Shimada K. [Long-term results after hepatectomy for hepatocellular carcinoma according to cancer stage]. Nihon Geka Gakkai Zasshi. 1998;99:219-222. [PubMed] [Cited in This Article: ] |
| 33. | A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 908] [Cited by in F6Publishing: 933] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 34. | Prospective validation of the CLIP score: A new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 362] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 35. | Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged Validation of a new prognostic system. Cancer. 2000;89:2266-2273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 36. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5490] [Cited by in F6Publishing: 5499] [Article Influence: 107.8] [Reference Citation Analysis (1)] |
| 37. | Matsumoto Y, Fujii H, Matsuda M, Kono H. Multicentric occurrence of hepatocellular carcinoma: diagnosis and clinical significance. J Hepatobiliary Pancreat Surg. 2001;8:435-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Ueno S, Tanabe G, Yoshida A, Yoshidome S, Takao S, Aikou T. Postoperative prediction of and strategy for metastatic recurrent hepatocellular carcinoma according to histologic activity of hepatitis. Cancer. 1999;86:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 39. | Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Sithinamsuwan P, Piratvisuth T, Tanomkiat W, Apakupakul N, Tongyoo S. Review of 336 patients with hepatocellular carcinoma at Songklanagarind Hospital. World J Gastroenterol. 2000;6:339-343. [PubMed] [Cited in This Article: ] |
| 41. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 595] [Cited by in F6Publishing: 642] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 42. | Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 43. | Tublin ME, Dodd GD, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR. Am J Roentgenol. 1997;168:719-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 216] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Dodd GD, Memel DS, Baron RL, Eichner L, Santiguida LA. Portal vein thrombosis in patients with cirrhosis: does sonographic detection of intrathrombus flow allow differentiation of benign and malignant thrombus AJR. Am J Roentgenol. 1995;165:573-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Cong WM. [Clinicopathologic features of small hepatocellular carcinoma--an analysis of ninety-three cases]. Zhonghua Zhongliu Zazhi. 1993;15:372-374. [PubMed] [Cited in This Article: ] |
| 46. | Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: Analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 48. | Yamanaka J, Yamanaka N, Nakasho K, Tanaka T, Ando T, Yasui C, Kuroda N, Takata M, Maeda S, Matsushita K. Clinicopathologic analysis of stage II-III hepatocellular carcinoma showing early massive recurrence after liver resection. J Gastroenterol Hepatol. 2000;15:1192-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Zhang ZJ, Wu MC, He J, Chen H, Yang JM, Yang GS, Cong WM, Zhang BH, Sheng F, Zong M. Influencing factors of disease free survival in hepatocellular carcinoma after hepatectomy. Zhonghua Yixue Zazhi. 2000;80:42-43. [Cited in This Article: ] |
| 50. | Chen MS, Li JQ, Li GH. [Clinical factors affecting prognosis of small hepatocellular carcinoma after hepatectomy]. Zhonghua Zhongliu Zazhi. 1994;16:225-227. [PubMed] [Cited in This Article: ] |
| 51. | Hanazaki K, Kajikawa S, Koide N, Adachi W, Amano J. Prognostic factors after hepatic resection for hepatocellular carcinoma with hepatitis C viral infection: univariate and multivariate analysis. Am J Gastroenterol. 2001;96:1243-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Ariizumi S, Takasaki K, Yamamoto M, Ohtsubo T, Saito A, Nakano M. Multicentric hepatocellular carcinomas tend to grow in more damaged segments of the liver. J Gastroenterol. 2000;35:441-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 53. | Donato MF, Arosio E, Del Ninno E, Ronchi G, Lampertico P, Morabito A, Balestrieri MR, Colombo M. High rates of hepatocellular carcinoma in cirrhotic patients with high liver cell proliferative activity. Hepatology. 2001;34:523-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Sangiovanni A, Colombo E, Radaelli F, Bortoli A, Bovo G, Casiraghi MA, Ceriani R, Roffi L, Redaelli A, Rossini A. Hepatocyte proliferation and risk of hepatocellular carcinoma in cirrhotic patients. Am J Gastroenterol. 2001;96:1575-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |