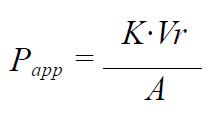Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.193
Revised: February 23, 2001
Accepted: March 1, 2001
Published online: April 15, 2001
AIM: To study the transepithelial transport characteristics of the polyamine putrescine in human intestinal Caco-2 cell monolayers to elucidate the mechanisms of the putrescine intestinal absorption.
METHODS: The transepithelial transport and the cellular accumulation of putrescine was measured using Caco-2 cell monolayers grown on permeable filters.
RESULTS: Transepithelial transport of putrescine in physiological concentrations ( > 0.5 mM) from the apical to basolateral side was linear. Intracellular accumulation of putrescine was higher in confluent than in fully differentiated Caco-2 cells, but still negligible (less than 0.5%) of the overall transport across the monolayers in apical to basolateral direction.EGF enhanced putrescine accumulation in Caco-2 cells by four fold, as well as putrescine conversion to spermidine and spermine by enhancing the activity of S adenosylmethionine decarboxylase. However, EGF did not have any significant influence on putrescine flux across the Caco- 2 cell monolayers. Excretion of putrescine from Caco-2 cells into the basolateral medium did not exceed 50 picomoles, while putrescine passive flux from the apical to the basolateral chamber, contributed hundreds of micromoles polyamines to the basolateral chamber.
CONCLUSION: Transepithelial transport of putrescine across Caco-2 cell monolayers occurs in passive diffusion, and is not influenced when epithelial cells are stimulated to proliferate by a potent mitogen such as EGF.
- Citation: Milovic V, Turchanowa L, Stein J, Caspary WF. Transepithelial transport of putrescine across monolayers of the human intestinal epithelial cell line, Caco-2. World J Gastroenterol 2001; 7(2): 193-197
- URL: https://www.wjgnet.com/1007-9327/full/v7/i2/193.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i2.193
Polyamines putrescine, spermidine and spermine are small aliphatic cations necessary for cell growth. If the cells are deprived from their polyamines they will stop dividing, resuming their proliferation rates after restoration of normal polyamine levels[1,2]. Polyamine content in the cell is tightly regulated: in addition to synthesis (via ornithine decarboxylase, synthesising putrescine from ornithine; and S-adenosylmethionine decarboxylase, an aminopropyl donor, synthesising spermidine and spermine), all mammalian cells are equipped with an efficient polyamine uptake system, regulated by mitogens[3-6] and capable of supplying the rapidly proliferating cells with increasing amounts of polyamines from the extracellular space.
Intestinal lumen is the main exogenous source of polyamines for the body. The prevailing polyamine in the intestinal lumen, putrescine, is present in human diet in high amounts[7,8]. In particular, food of plant origin contain excessive amounts of putrescine, while meat and meat products are rich in other two polyamines, spermidine and spermine. Postprandial putrescine concentrations in the duodenal and jejunal lumen can reach millimolar levels[9]. However, not longer than 2 h after meal, luminal polyamine content returns to baseline. Luminal putrescine is rapidly converted to metabolically active spermidine already in the intestinal wall[10,11]. However, surprisingly little is known about absorption mechanisms of luminal putrescine and its final metabolic fate, in spite of the findings that outlined the importance of luminal putrescine (and other polyamines) in both normal and neoplastic growth throughout the body. Luminal polyamines were found to support tumor growth[12], and are found to accumulate in tissues of high demand, i.e., high proliferation rate, such as neoplasia[13,14] or skeletal muscle stimulated to grow by clenbuterol[15].
To elucidate the mechanisms of putrescine absorption from the intestinal lumen, we used Caco-2 cells grown on permeable filters as an experimental model. Caco-2 cells originate from human colonic adenocarcinoma, but after two weeks in culture, spontaneously differentiate into an enterocyte-like phenotype[16]. These cells form monolayers with well-developed tight junctions[17], and have been evaluated in detail as a model to study both transcellular and paracellular transport of nutrients and drugs in the gut[17-20].
EGF (human recombinant, expressed in S. cerevisiae), unlabelled putrescine, spermidine and spermine were purchased from Sigma Chemie (Deisenhofen, Germany). [3H]-putrescine was obtained from Amersham Buchler (Braunschweig, Germany). Cell culture media and supplements were purchased from Gibco BRL (Eggenstein, Germany).
Cell culture Caco-2 cells were obtained from the German Cancer Research Centre, Heidelberg. Cells of passage number 49-56 were used. The cells were maintained in Dulbecco’s modified Eagle medium (DMEM) containing 4.5 g/L glucose and 25 mM Hepes, supplemented with 10% foetal calf serum, 100 U/mL benzylpenicillin and 10 mg/L streptomycin. The medium was changed every second day. The cells were routinely checked for Mycoplasma in monthly intervals.
For the experiments, the cells were expanded in tissue culture flasks (225 cm2 growth area), detached by treatment with 0.5 g/L trypsin and 0.2 g/L EDTA in PBS, and, to allow monolayers to be formed and uptake to be studied in conditions closely resembling this in the human intestinal epithelium, reseeded to 10 cm2 cell culture filters (ANOPORE; Nunc, Roskilde, Denmark); 2 × 106 cells were seeded to each plate. After 24 h, 1.5 mL of the medium was replaced with fresh medium and further maintenance was done as described above.
Putrescine uptake studies Cells were monitored for confluence by phase contrast microscopy. Confluence was reached at average after 6 or 7 days of culture. Confluent (day 7) or differentiated (day 14) Caco-2 cells were washed twice with PBS and synchronised in serum-free DMEM overnight. The studies on polyamine uptake into Caco-2 cells were done by adding 50 μM of each polyamine (0.1 μCi/mL) to the cell culture wells. After the desired period uptake was terminated by washing the cell monolayers twice with cold PBS to remove non-absorbed radioactivity from the cell surface. The filters were then removed by cutting using a sterile scalpel, and the cells were permeabilised with 1N NaOH, neutralised with 1 N HCl and the radioactivity was measured by standard liquid scintillation counting. In the experiments where a known enhancer of polyamine uptake into the cells, EGF was used, the procedure was carried out by the same manner except that the cells were incubated with 100 μg/L EGF for 12 h prior to the uptake experiments. Because EGF receptors in both confluent and differentiated Caco-2 cells are localised predominantly at the basolateral side[21], EGF was routinely added only to the basolateral chamber.
Permeability of putrescine across Caco-2 cell monolayers Transport studies using radiolabelled putrescine were performed on Caco-2 cells cultured on permeable filters. All experiments were carried out at 37 °C in serum-free DMEM under ‘sink’ conditions, as described by Artursson[18-20], using Caco-2 monolayers which were 14 days old. Transepithelial resistance was routinely measured using Millicell TER chamber (Millipore, Eschborn, Germany), and the value of 250 Ohm × cm 2 was considered sufficient to allow permeability studies.
In brief, all solutions were preheated to 37 °C and the experiments were performed at 37 °C ± 1 °C on a custom-built heating plate. The cell monolayers were washed with preheated DMEM and equilibrated in the same medium for 20-25 min prior to the transport experiments. The filters were then transferred to wells containing 1.2 mL fresh preheated DMEM. One millilitre DMEM containing radiolabelled test substance was added to the apical (“donor”) chamber. The [14C]-mannitol (MW 182, comparable to molecular weight of putrescine - 161.1) was used as a marker molecule to assess the integrity of the monolayers during the experiment. Radioactivity was used at concentrations of 10000 or 20000 Bq/mL for Caco-2 monolayers. Samples were taken from the apical solutions in order to measure the initial donor concentration. At four regular time intervals, the inserts were moved to new wells and samples were taken from the basolateral solution. All samples were analysed directly as described below.
The possible effect of putrescine adhesion to permeable supports was investigated by comparing the diffusion of [14C]-putrescine across cell-free supports. No retarded diffusion of [14C]-putrescine was observed, which showed that possible adhesion of putrescine to extracellular material could be neglected.
Analytical methods S-adenosylmethionine -decarb oxylase activity in Caco-2 cells was assayed as described before[22], by measuring the amount of 14CO2 liberated from S-adenosyl (carboxy 14C)-L-methionine. The final volume of incubation medium consisted of 50 mM Tris-HCl pH 7.2, 0.2 mmol/L S-adenosyl (carboxyl 14C)-L-methionine, 0.05 mmol/L pyridoxal-5-phosphate, 2.5 mmol/L DTT, and 0.1 mmol/L EDTA. The incubation period at 37 °C was 60 min. Radioactive samples were analysed using a liquid scintillation counter (Packard Instruments 1900CA TRI-CARB® ; Canberra Pacard Instruments, Downers Grove, IL). Protein was measured by Coomassie blue assay, by kits obtained from Biorad Laboratories GmbH (Munich, Germany).
Calculations The apparent permeability coefficient (Papp, cm/s) was determined according to the following equation[18-20]:
Math 1
where K is the steady state rate of change in concentration in the receiver chamber (Ct/C0) versus time (s), Ct is the concentration in the receiver compartment at the end of each time interval, C0 is the initial concentration in the apical chamber at each time interval (mole/mL), Vr is the volume of the receiver chamber (mL) and A is the surface area of the filter membrane (cm2).
Statistics The results were expressed as mean values ± SEM of four to six experiments. One-way ANOVA was used to compare means. A 95% probability was considered significant.
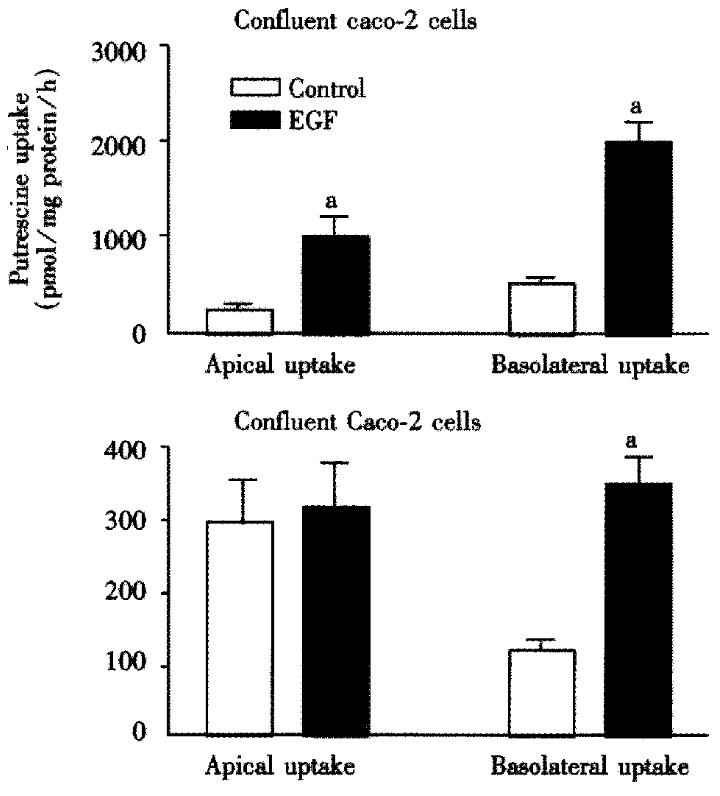
Caco-2 cell monolayers were incubated with and without 100 μg/L EGF for 12 h and then with 50 μM radiolabelled putrescine for different time intervals. Uptake rate of putrescine increased rapidly and was significantly higher in confluent than in differentiated Caco-2 cells (Figure 1). In confluent, 7 days old cells, EGF-induced putrescine uptake was significantly higher than in differentiated 14 days old cells, basolateral uptake being markedly higher than apical uptake when the cells were stimulated with EGF.
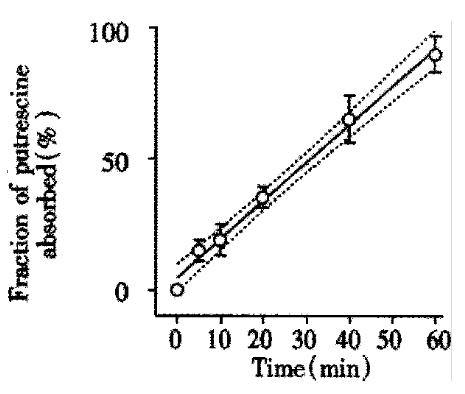
The transepithelial resistance in Caco-2 cell monolayers reached a plateau of 234 ± 12 Ohm × cm2 after approximately one week in culture and maintained this value for at least 40 days in culture, which was in agreement with previous findings[18-20]. Such monolayers of Caco-2 cells transported [14C]-mannitol with Papp of 22 ± 4 × 106 cm/s, which was another proof of their well developed tight junctions and full functional integrity. When physiological amounts of putrescine (100 μM; 0.1 μCi/mL) was added to the apical chamber and flux into the basolateral chamber measured over time, transport was linear. Almost the entire applied concentration diffused to the basolateral chamber within a 60 min experimental period (Figure 2).
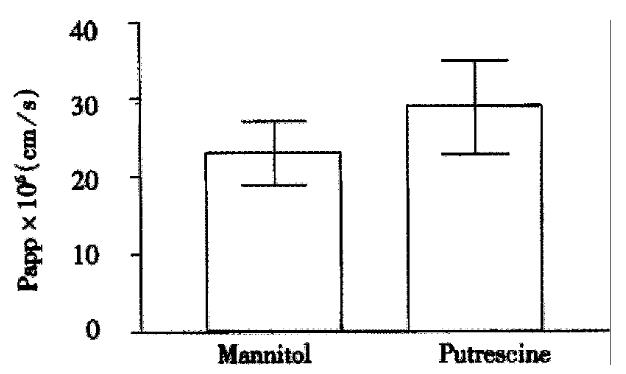
Apparent permeability constants (Papp) for putrescine was in the range of 18-35 × 106 cm/s, which was similar to Papp of the standard marker for paracellular permeability, mannitol (Figure 3). There was no change in Papp values when the cells were incubated in a balanced salt solution without glucose (data not shown).
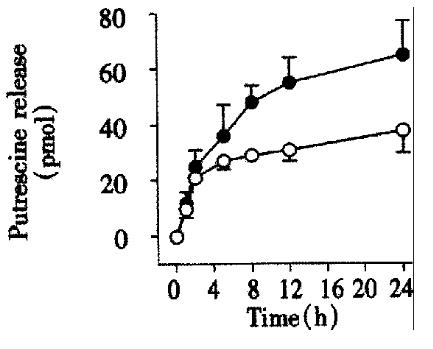
A 12 h incubation with 100 μg/L EGF stimulated not only putrescine uptake in CaCo-2 cells (Figure 1), but also its conversion to spermidine and spermine. This conclusion was based on the finding that EGF stimulated the activity of S-adenosylmethionine decarboxylase, the enzyme responsible for conversion of putrescine to spermidine and spermine by approx imately 90% (control: 114 ± 9 pmol CO2/mg protein, vs EGF: 201 ± 8 pmol CO2/mg protein, P < 0.01). To investigate whether an increase of putrescine uptake in the intestinal epithelium can be more profoundly increased by a potent growth stimulus such as EGF (and therefore its general absorption influenced), we preloaded control and EGF-treated Caco-2 cell monolayers with physiological concentrations of putrescine (100 μmol), allowed to incubate for 2 h (time period previously shown to be sufficient for maximal uptake)[5], then washed the monolayers with PBS to remove non-absorbed radioactivity, and transferred to radioactivity-free cell culture wells. Release of the radioactivity originating from exogenous putrescine to the basolateral chamber in both control and EGF-treated cells was initially rapid, reaching plateau not earlier than after 6 h incubation (Figure 4). Up to 24 h only minute amounts of the radioactivity was released across the basolateral membrane, the values not exceeding 45 pmol; EGF did not have major influence on putrescine release.
Our results indicate that putrescine transport through the intestinal epithelial barrier is linear, the levels taken up by the epithelial cells not exceeding 0.5% of those passively transported across the intestinal epithelium. Treatment with EGF, known to up-regulate putrescine uptake in Caco-2 cells, did enhance putrescine accumulation in Caco-2 cells, but this had no overall impact on net transport of putrescine across the intestinal epithelium, in concentrations shown to exist postprandially in the intestinal lumen[9]. In spite of all limitations of the model used (i.e., absence of subepithelial tissue, inability to measure possible rapid metabolisation of absorbed putrescine in the intestinal wall), our data lead to the conclusion that, in the gut, putrescine is absorbed exclusively by passive diffusion.
A number of studies done in different experimental models show that polyamines rapidly disappear from the intestinal lumen after meal and are rapidly distributed in the body, being directed to tissues with highest demand, i.e. with the highest proliferation rate[23]. Our data may provide an explanation about the possible mechanism for the rapid disappearance of dietary putrescine from the gut lumen. At physiological pH putrescine is fully charged. Small, charged and hydrophilic molecules are usually absorbed via the paracellular pathway, and their transport is additionally facilitated by solvent drug[24]. Indeed, in our experimental model, permeability constant for putrescine did not significantly differ from this of a distinct paracellular marker sized similar to putrescine, mannitol (Figure 2).
The presence of several hundreds of micromoles putrescine in the postprandial duodenal and jejunal lumen, while its concentration in blood and tissues hardly exceeds tens of micromoles, clearly rises a need to offer an explanation for such a high supply and low utilisation of this polyamine in the body. Excess luminal putrescine has recently been attributed as an instant energy source in the gut[25]. Furthermore, endothelial cells in the gut are rich in diamine oxidase, the enzyme able to degrade excess putrescine to succinate and GABA[26]. In the only study of this kind done in humans, disappearance of putrescine from the human intestinal lumen was found to be linear; however, the amounts of putrescine itself, shortly after the putrescine-rich test meal, showed that no free putrescine was detectable in blood. Instead, its acetylated derivative was rapidly and markedly increased, while the amounts of spermidine and spermine increased less rapidly but progressively[27]. All these data may fit well into the hypothesis that putrescine, as well as other two polyamines, cross the intestinal epithelial barrier passively, and that they are then both rapidly metabolised in situ and used as an instant energy source, or, also rapidly, metabolised into spermidine and spermine and then transported throughout the body.
Apart from transport across the intestinal epithelium, small amounts of putrescine in our study were also taken up by enterocyte-like Caco-2 cells. Putrescine uptake was also stimulated by EGF. Perhaps surprisingly, this uptake was higher across the basolateral then the apical membrane, and the difference was further increased after stimulation with EGF. The role of basolateral polyamine uptake when the gut is stimulated to grow has been described before[28-30], and putative polyamine transporters at the basolateral membrane of the enterocyte has been well characterised at the biochemical level[31,32]. Our data might also additionally confirm an interesting hypothesis that polyamines, via local circulation in the intestinal wall, do reach the enterocyte via their basolateral membranes rather than directly, via the brush border membrane of the enterocyte[33].
In conclusion, our data show that putrescine crosses the intestinal epithelial barrier passively, while only minor amounts are taken up by intestinal epithelial cells. Our data provide an explanation for a known rapid disappearance of dietary polyamines from the intestinal lumen, and their rapid distribution in the body, where they then exert their known growth-related actions.
The data were presented in part at the European Intestinal Transport Group meeting in Lecce (Italy), in May 1995. The authors are indebted to Professor Per Artursson (Department of Pharmaceutics, Uppsala University, Sweden) for his help in establishing the Caco-2 cell model, to Dr Gerard M. Murphy and Professor R. Hermon Dowling (UMDS, Guy’s Hospital, London, UK) for critical discussions, and Caroline von Sch¢ippenthau (born Deubner) for help in experimental work. The study was supported by a postdoctoral research fellowship of the Alexander von Humboldt Foundation (to Dr Vladan Milovic).
Edited by Ma JY
| 1. | Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 711] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Dandrifosse G, Deloyer P, Grandfils C, Loret S, Peulen O, Dandrifosse AC. [Statement on polyamines]. Rev Med Liege. 1999;54:175-183. [PubMed] |
| 3. | Dowling RH, Hosomi M, Stace NH, Lirussi F, Miazza B, Levan H, Murphy GM. Hormones and polyamines in intestinal and pancreatic adaptation. Scand J Gastroenterol Suppl. 1985;112:84-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Goodlad RA, Gregory H, Wright NA. Is polyamine synthesis involved in the proliferative response of the intestinal epithelium to urogastrone-epidermal growth factor. Clin Sci (Lond). 1989;76:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Milovic V, Deubner C, Zeuzem S, Piiper A, Caspary WF, Stein J. EGF stimulates polyamine uptake in Caco-2 cells. Biochem Biophys Res Commun. 1995;206:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Milovic V, Stein J, Odera G, Gilani S, Murphy GM. Low-dose deoxycholic acid stimulates putrescine uptake in colon cancer cells (Caco-2). Cancer Lett. 2000;154:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Bardocz S. The role of dietary polyamines. Eur J Clin Nutr. 1993;47:683-690. [PubMed] |
| 8. | Bardócz S, Duguid TJ, Brown DS, Grant G, Pusztai A, White A, Ralph A. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 182] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Sawada Y, Pereira SP, Murphy GM, Dowling RH. Origins of intes-tinal luminal polyamines in man. Gut. 1994;35:S20. |
| 10. | Hosomi M, Lirussi F, Stace NH, Vaja S, Murphy GM, Dowling RH. Mucosal polyamine profile in normal and adapting (hypo and hyperplastic) intestine: effects of DFMO treatment. Gut. 1987;28 Suppl:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Osborne DL, Seidel ER. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am J Physiol. 1990;258:G576-G584. [PubMed] |
| 12. | Sarhan S, Knodgen B, Seiler N. The gastrointestinal tract as polyamine source for tumor growth. Anticancer Res. 1989;9:215-223. [PubMed] |
| 13. | Seiler N, Sarhan S, Grauffel C, Jones R, Knödgen B, Moulinoux JP. Endogenous and exogenous polyamines in support of tumor growth. Cancer Res. 1990;50:5077-5083. [PubMed] |
| 14. | Löser C, Torff L, Fölsch UR. Uptake of extracellular, dietary putrescine is an important regulatory mechanism of intracellular polyamine metabolism during camostate-induced pancreatic growth in rats. Dig Dis Sci. 1997;42:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Bardocz S, Brown DS, Grant G, Pusztai A, Stewart JC, Palmer RM. Effect of the beta-adrenoceptor agonist clenbuterol and phytohaemagglutinin on growth, protein synthesis and polyamine metabolism of tissues of the rat. Br J Pharmacol. 1992;106:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Pinto M, Robine Leon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon Assman P, Haffen K, Fogh J. Enterocyte like differentiation and polarisation of the human colon carcinoma cell line Caco 2 in culture. Biol Cell. 1983;47:323-330. |
| 17. | Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736-749. [PubMed] |
| 18. | Artursson P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J Pharm Sci. 1990;79:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 643] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1396] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 20. | Artursson P. Cell cultures as models for drug absorption across the intestinal mucosa. Crit Rev Ther Drug Carrier Syst. 1991;8:305-330. [PubMed] |
| 21. | Hidalgo IJ, Kato A, Borchardt RT. Binding of epidermal growth factor by human colon carcinoma cell (Caco-2) monolayers. Biochem Biophys Res Commun. 1989;160:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Milovic V, Turhanowa L, Fares FA, Lerner A, Caspary WF, Stein J. S-adenosylmethionine decarboxylase activity and utilization of exogenous putrescine are enhanced in colon cancer cells stimulated to grow by EGF. Z Gastroenterol. 1998;36:947-954. [PubMed] |
| 23. | Milovic V, Murphy GM, Bauske R, Stein J. Characterisation and regulation of polyamine uptake in the gut. In: Morgan DML, White A, Sanchez Jimenez F,Bardocz S, eds. Biologically active amines in food Vol. IV, COST 917 European Commission. 2000;74-79. |
| 24. | Chang EB, Rao MC. Intestinal water and electrolyte transport. In: Johnson LR, ed. Physiology of the gastrointestinal tract. 3rd Ed. New York: Raven Press. 1994;2027-2081. |
| 25. | Bardócz S, Grant G, Brown DS, Pusztai A. Putrescine as a source of instant energy in the small intestine of the rat. Gut. 1998;42:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Rokkas T, Vaja S, Taylor P, Murphy GM, Dowling RH. Effect of intestinal diamine oxidase (DAO) depletion by heparin on mucosal polyamine metabolism. Digestion. 1990;46 Suppl 2:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Milovic V, Odera G, Murphy GM, Dowling RH. Jejunal putrescine absorption and the "pharmacokinetics"/biotransformation of ingested putrescine in humans. Gut. 1997;41:A62. |
| 28. | Bardocz S, Brown DS, Grant G, Pusztai A. Luminal and basolateral polyamine uptake by rat small intestine stimulated to grow by Phaseolus vulgaris lectin phytohaemagglutinin in vivo. Biochim Biophys Acta. 1990;1034:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Bardócz S, Grant G, Brown DS, Ewen SW, Nevison I, Pusztai A. Polyamine metabolism and uptake during Phaseolus vulgaris lectin, PHA-induced growth of rat small intestine. Digestion. 1990;46 Suppl 2:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Bardócz S, Grant G, Brown DS, Ewen SW, Stewart JC, Pusztai A. Effect of fasting and refeeding on basolateral polyamine uptake and metabolism by the rat small bowel. Digestion. 1991;50:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Milovic V, Stein J, Piiper A, Gerhard R, Zeuzem S, Caspary WF. Characterization of putrescine transport across the intestinal epithelium: study using isolated brush border and basolateral membrane vesicles of the enterocyte. Eur J Clin Invest. 1995;25:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Milovic V, Caspary WF, Stein J. Polyamine uptake across the basolateral membrane of the enterocyte is mediated by a high-affinity carrier: a study using isolated basolateral membrane vesicles. Digestion. 1998;59:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | McCormack SA, Johnson LR. Role of polyamines in gastrointestinal mucosal growth. Am J Physiol. 1991;260:G795-G806. [PubMed] |