Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.304
Revised: June 6, 1999
Accepted: June 25, 1999
Published online: April 15, 2000
- Citation: Xie B, He SW, Wang XD. Effect of gastrin on protein kinase C and its subtype in human colon cancer cell line SW480. World J Gastroenterol 2000; 6(2): 304-306
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/304.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.304
Gastrin is a trophic gastrointestinal hormone which is secreted by G cell. Gastrin has long been considered a growth stimulatory hormone for mucosa of the gastrointestinal tract[1]. The growth responses of certain colorectal cancer cells, and xenografts, can be stimulated by endogenous gastrin[2]. Protein kinase C (PKC) is a family of isozymes that plays a crucial role in transducing signals of many hormones, growth peptides, neurotransmitters, and its activation is crucial in tumor promotion[3]. PKC is also involved in regulating cellular prolife ration[4]. On the basis of gastrin’s effect on the growth of human colon cancer cell, we further studied its effect on PKC and its subtype so as to elucidate the molecular basis in signal transduction mechanisms regulating SW480 cell proliferation and provide an experimental evidence of antisignaling therapy for patients with colon cancer.
Pentagastrin (PG) was purchased from Shanghai Li Zhu Biochemical Co., proglumide (PGL) from Shanghai No.11 Pharmaceutical Factory, PS, Histone III-s from Sigma Chemical Co., and γ-32P-ATP from Beijing Yahui Co. Human colon cancer line SW480 was obtained from Silon-kittelng Cancer Center (American), and PKC-α, β, γ cDNA probes from Biological Deparment of Beijing Teacher’s University. Dig-labelling and detection kit was purchased from Boehringer Mannheim Co.
Cell in culture Cells were maintained in culture medium (RPMI 1640), containing 100 mL/L fetal calf serum in a humidified atmosphere of 950 mL/L air and 50 mL/L CO2 at 37 °C.
Extraction of PKC from SW480 Cells were incubated with or without stimulating agents at the desired time intervals (1 min, 2 min and 5 min), and the reaction was terminated by the rapid addition of an icecold homogenizing buffer. The preparations were centrifuged at 200 × g for 5 min at 4 °C. The supernatants were discarded and the pellets were suspended in chilled buffer and were sonicated on ice for 45 min at 4 °C. The resulting supernatants (cytosolic fraction) were removed and kept on ice. The pellets were resuspended and centrifuged at 100000 × g for 45 min at 4 °C, and the supernatant represented the membrane fractions. The cytosolic or membrane fractions were used directly.
Measurement of PKC activity PKC was assayed by measuring the incorporation of 32P from [γ-32P]ATP into a specific peptide substrate (Sigma). The reaction was then terminated, and aliquots from each sample were spotted on specific binding papers. The papers were allowed to soak, and then were washed twice individually and processed for counting as described previously[5]. Protein content of each sample was measured by the Bradford method, using bovine serum albumin as a standard[6]. The assays were constantly made for linear range of enzyme activity with regard to protein concentration and incubation condition. Assays were performed in duplicate and the results were expressed as picomoles of 32P incorporrated in the peptide substrate/minute/mg protein.
Preparation of in situ hybridization samples Cells (approximately 1 × 106/mL), which were plated and cultured in 24-well tissue culture plates (each well had a coverslip on the bottom) for 48 h, and were incubated in the presence of PG (25 mg/L) for 24 h. Control group cells were incubated with RPMI 1640 containing 10% FCS instead of PG. Cells were incubated for 24 h, then the supernatants were discarded. The remains were washed with D-Hanks buffer for three times, fixed with formaldehyde for 5 min and stored in 4 °C ice-box.
Dig-labelling and detection of probe The probe was labelled by random hexade primer (RHP) method using Dig-labelling and detection kit-(Boehringer Mannheim Co.)
In situ hybridization of samples The procedures with some modifications were mainly as follows[7] : the samples were baked at 85 °C for 5 min, deparaffinized and rehydrated in graded alcohol, then put into 200 mL/L cold acetic acid and at 4 °C for 20 min. Hybridization buffer with the concentration of the probe (1 mg/L) was added to the slides and incubated at 42 °C for 20 h. After washed thoroughly, the slides were darkly developed with NBT-BCIP at room temperature for 0.5 h-1 h, and then slightly stained with 0.5% methyl green, and mounted by glycerine-gelatin.
Controls The slides were treated with 20 mg/L RNase at 37 °C for 30 min before hybridization. No probe was put into the prehybridization buffer.
A one-way analysis of variance (ANOVA, Excel 6.0 band) was used to test for statistical significance between paired or unpaired data. All values were expressed as mean ± SEM and P < 0.05 was considered statistically significant and P < 0.01 was considered as very significant difference.
We examined the effect of gastrin on PKC activity (including cytosol and membrane) in SW480 cells for the desired time intervals. The PKC activity of membrane was increased obviously and the PKC activity of cytosol was decreased when cells were treated by PG for 1 min. After 2 min, the PKC activity of membrane and cytosol was still higher than in control group, and after 5 min it turned basically to the previous level (Table 1).
To investigate whether PKC activity is part of a receptor-mediated pathway, cells were incubated with the gastrin receptor antagonist PGL. The result shows that PGL (32 mg/L) almost abolished the effect of PG (25 mg/L) on PKC activity in SW480 cell (Table 2).
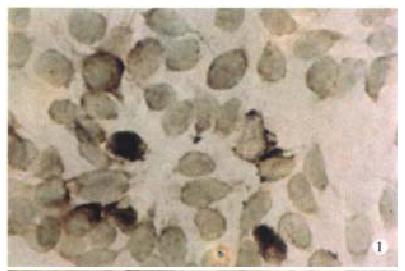
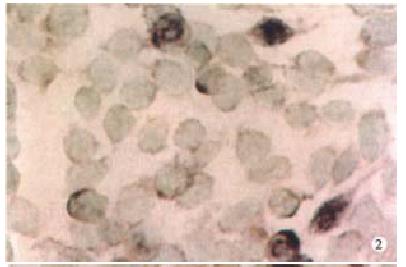
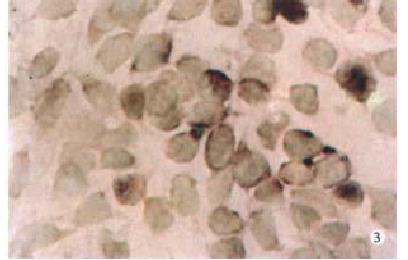
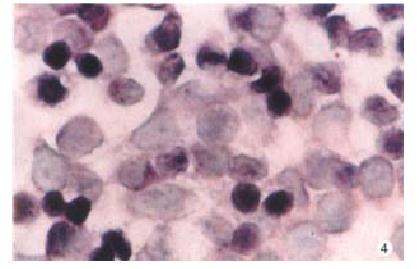
Expression of PKC-α, β, γ-mRNA all existed in SW480 cells at some extent, but after PG treatment, the expression of PKC-βmRNA was increased more than that of α, γmRNA (Figure 1, Figure 2, Figure 3, Figure 4).
It is well known that the stimulation of PKC is a critical step in signal transduction pathways, and the translocation of PKC from cytosol to membrane was recognized as a symbol of PKC activiation. Gastrin can increase the translocation effect[8,9]. Differences in the distribution of PKC activities were reported in certain cultured cell lines, such as human stomach, colon and breast cancers due to the expression of vario us PKC subtypes[10]. It is clear that cell growth is regulated by various growth factors through their specific receptors and receptor-linked signal transduction pathways[11], such as the cAMP pathway, the tyrosine kinase pathway, PI hydrolysis, or mobilization of intracellular calcium. It has been observed that gastrin exerted a growth-promoting effect on colonic epithelial cells in vitro[5], and the effect was mediated by the activation of PKC. But the mechanisms of gastrin as to how to regulate growth have not been clarified.
Our data showed that PG decreased the activity of PKC in the cytosol of cancer cells, while increased the activity of PKC in the membrane, and that the effect of PG on PKC possessed time-dependent character. In this experiment, we have also demonstrated that PGL, a gastrin receptor antagonist, blocked the effect of PG on PKC activation. The result indicated that PG stimulated PKC through a specific receptor-mediated pathway. It is clear that the signal pathway through PKC appears to be absolutely necessary for the control of gene expression and the cell cycle in the nucleus[12]. Persons et al[13] reported that transfected NIH3T3 cells carrying high-level expression vectors of rat brain PKC-1cDNA displayed enhanced tumorigenicity when inoculated into nude mice. Yang XH et al[14] reported that overexpression of PKC-β resulted in a high rate of cell growth. To further elucidate the role of specific PKC subtype in growth control and tumor promotion, we have observed the effect of gastrin on expression of PKC-α, β, γmRNA. The result showed that the differences among expression of PKC-α, β, γmRNA originally existed in SW480 cells, but after gastrin treatment, the expression of PKC-βmRNA was increased more than that of PKC-α, γmRNA. These results indicated that PKC-β may play a critical role in cell proliferation.
Chemo-endocrine chemotherapy with gastrin receptor antagonist, such as proglumide, can prevent the recurrence after resection of liver metastasis in colorectal cancer[15]. In accordance with the role of PKC in gastrin stimulating cancer cells proliferation, PKC antagonists, such as staurosproine and bryostatin, have been used to treat colon cancer. So we can design and choose “signal drugs” for antisignaling therapy for patients with colon cancer.
Edited by Ma JY
| 1. | Johnson LR. The trophic action of gastrointestinal hormones. Gastroenterology. 1976;70:278-288. [PubMed] |
| 2. | Morris DL, Watson SA, Durrant LG, Harrison JD. Hormonal control of gastric and colorectal cancer in man. Gut. 1989;30:425-429. [PubMed] |
| 3. | O'Brian CA, Ward NE. Biology of the protein kinase C family. Cancer Metastasis Rev. 1989;8:199-214. [PubMed] |
| 4. | Craven PA, DeRubertis FR. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation by unsaturated fatty acids. Gastroenterology. 1988;95:676-685. [PubMed] |
| 5. | Yassin RR, Murthy SN. Possible involvement of protein kinase C in mediating gastrin-induced response in rat colonic epithelium. Peptides. 1991;12:925-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157401] [Article Influence: 3212.3] [Reference Citation Analysis (0)] |
| 7. | Cai WQ, Wang BY. Practical immunocytochemistry and nucleic acid hybridization techniques. Ed 1. Chengdu: SichuanScientific Technical Press 1994; 121-124. |
| 8. | Yassin RR, Clearfield HR, Little KM. Gastrin's trophic effect in the colon: identification of a signaling pathway mediated by protein kinase C. Peptides. 1993;14:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Rozengurt E, Rodriguez-Pena A, Coombs M, Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci USA. 1984;81:5748-5752. [PubMed] |
| 10. | Hashimoto Y, Chida K, Huang M, Katayama M, Nishihira T, Kuroki T. Levels of protein kinase C activity in human gastrointestinal cancers. Biochem Biophys Res Commun. 1989;163:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Rozengurt E. Neuropeptides as cellular growth factors: role of multiple signalling pathways. Eur J Clin Invest. 1991;21:123-134. [PubMed] |
| 12. | Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607-614. [PubMed] |
| 13. | Persons DA, Wilkison WO, Bell RM, Finn OJ. Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell. 1988;52:447-458. [PubMed] |
| 14. | Yang XH, Li H, Liu HT. Study of PKC-βI overexpression NRK cell growth disorder. Kexue Tongbao. 1993;38:2186-2189. |
| 15. | Kameyama M, Nakamori S, Imaoka S, Yasuda T, Nakano H, Ohigashi H, Hiratsuka M, Sasaki Y, Kabuto T, Ishikawa O. [Adjuvant chemo-endocrine chemotherapy with gastrin antagonist after resection of liver metastasis in colorectal cancer]. Gan To Kagaku Ryoho. 1994;21:2169-2171. [PubMed] |