Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.252
Revised: December 6, 1999
Accepted: December 11, 1999
Published online: April 15, 2000
AIM: To evaluate the possibility of using cultured human hepatocytes as a bridge between bioartificial liver and liver transplantation.
METHODS: In this experiment, the efficacy of extracorporeal bio artificial liver support system (EBLSS) consisting of spheriodal human liver cells and cultured hepatocytes supernatant was assessed in vivo using galactosamine induced rabbit model of fulminant hepatic failure.
RESULTS: There was no difference of survival between the two groups of rabbits, but in the supported rabbits serum alanine aminotransferase, total bilirubin and creatinine were significantly lower and hepatocyte necrosis was markedly milder than those in control animals. In addition, a good viability of human liver cells was noted after the experiment.
CONCLUSION: EBLSS plays a biologic role in maintaining and compensating the function of the liver.
- Citation: Wang YJ, Li MD, Wang YM, Chen GZ, Lu GD, Tan ZX. Effect of extracorporeal bioartificial liver support system on fulminant hepatic failure rabbits. World J Gastroenterol 2000; 6(2): 252-254
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.252
The recent development of bioartificial liver support system containing hepatocytes is an important event in the research of the artificial liver[1]. This system possesses the potential metabolic and synthetic functions of the liver and may provide certain nutrients and cytokines necessary for the regeneration of liver tissues[2,3]. Further development is expected in the research of the bioartificial liver to provide a new therapeutic procedure in the treatment of fulminant hepatic failure. Based on the studies of isolation and culture of human liver cells, we established an extracorporeal bioartificial liver support system (EBLSS) with cultured human hepatocytes and hollow fiber bioreactor. Its support efficiency was investigated preliminarily in vivo with animal model of hepatic failure.
Male and female rabbits weighing between 2 kg and 3 kg were provided by the Experimental Animal Center of Third Military Medical University. All animals were allowed free access to food and water.
A model of fulminant hepatic failure (FHF) in the rabbits was prepared by the method of Blitzer et al[4] with slight modifications. D-Galactosamine (D-Gal, purchased from Chongqing University) at a lethality dose of 1.2 g/kg of body weight was dissolved in 9 mL of 50 g/L dextrose in water and pH was adjusted to 6.8 with sodium hydroxide. The solution was then given intravenously over 5 min via ear vein.
The bioartificial liver system consisted of liver cells, hollow-fiber bioreactor and circulating unit. The cells were harvested from 4 mo old human fetal liver by a two step collagenase perfusion technique modified from the method of Seglen[5] and the hepatocytes and liver nonparenchymal cells were obtained by centrifugation at 50 × g for 3 min and 500 × g for 3 min respectively. Cell viability was initially > 96% for all devices assessed by trypan blue exclusion and they were successfully cultured as multicellular spheroids with a synthetic technique. About 1 × 108 viable cells were placed onto the outer space of the hollow fiber bioreactor. The hollow fibers (porous, 0.2 μm) were polysulfone with a 100 kDa nominal molecular weight cutoff and a 1128 cm2 surface area. Thirty mL anti-coagulate blood came from normal rabbit was perfused into the intracapillary space of hollow fiber bioreactor and the circulatory tube. A roller pump (Millipore ultrafiltration device) was used to circulate blood and a heater was used to maintain the animal blood and bioreactor temperature at 37 °C-39 °C. With this condition the system was ready for in vitro application.
The experimental animals were divided into two groups: group I animals (n = 5) were treated with EBLLS inoculated with viable liver cells; group II, animals (n = 5) were treated as control with EBLLS but without cells. Animals in both groups were anesthetized by pentobarbital (0.03 g/kg, intravenously) and femoral artery and vein catheters were placed before experiment. Four hours after the induction of FHF, the femoral artery and vein was cannulated for EBLSS access. Hemoperfusion was through the EBLSS at a rate of 15 to 20 mL/min. Heparin was administered at 150 U/kg firstly and at 50 U/kg every 30 min. Perfusion was carried out for 4 h. About 15 mL supernatant of cultured hepatocyte and liver nonparenchymal cells was administered into the extracapillary space for an assisting treatment during the experiments.
Blood samples were obtained at the initiation of the EBLSS hemoperfusion, during the treatment and at hourly intervals for 5 h after liver support. Serum alanine aminotransferase (ALT), total bilirubin (TB) and creatinine (Cr) were determined in the clinical laboratory using a Beckman CX-7 autoanalyzer (Beckman Instruments, Inc., Fullerton, Calif.) by standard methods.
Liver biopsy specimens were obtained from each of the animal postmortem and fixed in 10% formalin. Histological analyses were carried out in the pathological laboratory using standard procedures. The liver cell spheroids loaded in EBLSS were recollected and dispersion by 0.01% pancreatin and 0.1 mmol/L EDTA. Their viability was determined again by trypan blue exclusion. The rate of adherence to dish coated collagen was obtained by phase contrast microscope after 24 h of normal culture.
The survival time of FHF rabbits in the control group was 9.2 h, 14.6 h, 15.7 h, 24.2 h and 34.1 h (19.6 h ± 9.7 h). In EBLSS support group, besides one animal which died 10 min after the initiation of artificial support, the survival time was 11.4 h, 14.9 h, 24.2 h and 25.1 h respectively (18.9 h ± 6.8 h). There was no difference between the two groups (P > 0.05).
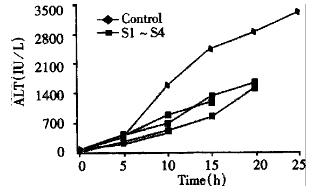
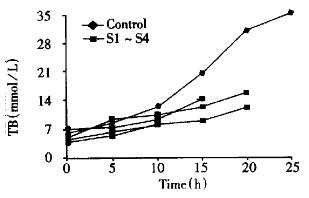
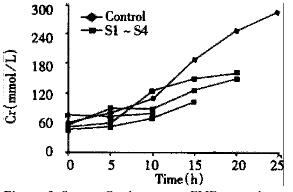
In both groups of animals, there was a progressive increase in the concentration of serum ALT, TB and Cr in 10 h after injection of D-Gal, but there was no statistical difference. Afterwards, a significant increase of serum ALT, TB and Cr was observed in control group. At the same time phase, serum ALT, TB and Cr were also increased in EBLSS support group, but the extent of increment was small relatively (Figure 1, Figure 2, Figure 3).
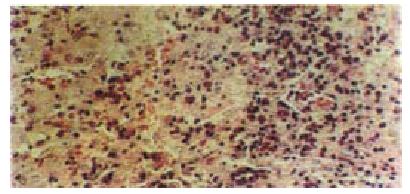
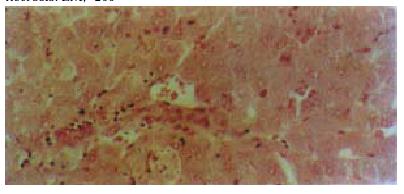
Histological examination of liver biopsy specimens obtained from the control rabbits demonstrated characteristic of classic acute hepatic necrosis, which contained multiple areas of lytic necrosis, disappearance of lobule construction, dissociation of hepatic cords and a large number of inflammatory cells infiltrated into the portal triads. In artificial liver support group, besides the large area of necrosis observed only in one animal, hepatocyte degeneration and focal necrosis were the main manifestation in the rest animals. The regeneration of hepatocytes was observed easily in this group (Figure 4, Figure 5).
Both liver cell viability and spheroids adherence rate were decreased by 13.1% and 26.2% respectively after the artificial liver support as compared with those before the experiment.
Medical treatment of fulminant hepatic failure is still not satisfactory, and the mortality is about 90%. It has been recognized that the liver transplantation is the only process in saving these patients. However, there is a shortage of donors and most patients have no sufficient time in waiting for the emergency transplantation. Therefore, many investigators devote their efforts to the development of artificial liver device as a liver support system to help maintain patients in alive and intact mentality until an organ donor becomes available. The liver is known as a complex metabolic organ with multi-functions essential for surviving, including gluconeogenesis, synthesis of blood proteins amino acid metabolism, urea synthesis, lipid metabolism, drug biotransformation, and removal of wastage. So the early artificial liver systems such as hemodialysis, hemadsorption and charcoal hemoperfusion that had limited purifying function can not bring a satisfactory result to these patients[6]. A bioartificial liver comprising viable hepatocytes on a mechanical support has been considered to provide these essential functions more likely than a purely mechanical device[7]. Up to now, a lot of data show that this new bioartificial liver system not only possesses the ability of specific detoxication and metabolism but also has synthetic functions[8,9].
Bioartificial liver system could maintain a suitable internal enviorment for the FHF patients to wait for the chance of liver transplantation.
In this study, after the human liver cells were isolated by an alternative extracorporeal two step perfusion method with a good viability, we succeeded in carrying out the aggregate culture of hepatocytes and nonperenchymal cells. Subsequently, we place the multicellular spheroids and supernatant of cultured hepatocyte into a hollow fiber bioreactor to form a simple EBLSS and its liver support function was assessed in vivo by D-Gal-induced rabbit model with fulminant hepatic failure. The biochemical results show that the increase of serum ALT, TB and Cr was restrained effectively in support group animals. At the same time, we found the pathological change of supported animals was markedly improved and active regeneration of hepatocytes was easily seen in these animals. These data indicate that this EBLSS possesses the effect of liver support to the fulminant hepatic failure animal in certain extents. The results of EBLSS in vitro show that it protects the cultured hepatocyte from injury by the D-Gal induced toxic metabolites and promotes the regeneration of hepatocytes by the substances afforded from the hepatocytes and supernatant of cultural medium. Nevertheless, the effectiveness of EBLSS has not been satisfactorily demon strated in in vivo animal experiment due to the difficulty in establishing extracorporeal circulation and maintaining a stable experimental condition, and because the animal is hard to endure the heavy hemodynamic burder.
Edited by You DY and Ma JY
| 1. | Butler A, Friend PJ. Novel strategies for liver support in acute liver failure. Br Med Bull. 1997;53:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Hughes RD, Williams R. Use of bioartificial and artificial liver support devices. Semin Liver Dis. 1996;16:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Gerlach J, Ziemer R, Neuhaus P. Fulminant liver failure: relevance of extracorporeal hybrid liver support systems. Int J Artif Organs. 1996;19:7-13. [PubMed] |
| 4. | Blitzer BL, Waggoner JG, Jones EA, Gralnick HR, Towne D, Butler J, Weise V, Kopin IJ, Walters I, Teychenne PF. A model of fulminant hepatic failure in the rabbit. Gastroenterology. 1978;74:664-671. [PubMed] |
| 5. | Wang YJ, Li MD, Wang YM, Nie QH, Chen GZ. Experimental study of bioartificial liver with cultured human liver cells. World J Gastroenterol. 1999;5:135-137. [PubMed] |
| 6. | Nyberg SL, Peshwa MV, Payne WD, Hu WS, Cerra FB. Evolution of the bioartificial liver: the need for randomized clinical trials. Am J Surg. 1993;166:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Watanabe FD, Demetriou AA. Nonsurgical management of fulminant hepatic failure. Curr Opin Gastroenterol. 1996;12:231-236. [DOI] [Full Text] |
| 8. | Watanabe FD, Shackleton CR, Cohen SM, Goldman DE, Arnaout WS, Hewitt W, Colquhoun SD, Fong TL, Vierling JM, Busuttil RW. Treatment of acetaminophen-induced fulminant hepatic failure with a bioartificial liver. Transplant Proc. 1997;29:487-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hughes RD, Williams R. Assessment of bioartificial liver support in acute liver failure. Int J Artif Organs. 1996;19:3-6. [PubMed] |
| 10. | Wang Y, Li M, Wang Y. [Study on the biologic function of extracorporeal bioartificial liver support system]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 1999;16:143-146. [PubMed] |