Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.157
Revised: March 1, 2000
Accepted: March 5, 2000
Published online: April 15, 2000
- Citation: Tiscornia OM, Hamamura S, Lehmann ES, Otero G, Waisman H, Tiscornia-Wasserman P, Bank S. Biliary acute pancreatitis: A review. World J Gastroenterol 2000; 6(2): 157-168
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.157
It is axiomatic that the most effective and soundly based plan of treatment of any disorder is one aimed at the mechanism or mechanisms responsible for its development[1]. This basic notion, coupled with recent reports[2-11] in which, surprisingly there is a total lack of reference to the probable involvement of autonomic-arc-reflexes in the physiopathogenesis of biliary acute pancreatitis have prompted this presentation. Undoubtedly, this disease entity has numerous causes, an obscure physiopathology, few effective remedies, and, often, an unpredictable outcome. At the turn of the century, Opie[12,13] brought to light the association between gallstone migration and acute pancreatitis. When referring to the intimate evolving process, most authors steadily adhere to the Opie’s theories: chemical and physical. The former is linked to the common-channel or biliary-reflux mechanism. The latter, to the stone-elicited Wirsung duct obstruction, with the resultant hypertension in the pancreatic ductal tree. What is indeed surprising is the lack of speculation on the subsequent steps that necessarily must follow the initial chemical and/or physical injuries that end up in an episode of acute pancreatitis. The core of our contention is that the pathophysiology of biliary acute pancreatitis is centered on two basic mechanisms: the activation of autonomic arc reflexes and the disruption of the entero-pancreatic feedback loop. Superimposed on the aforementioned pivotal processes we must consider, in some cases, the aggravating role of alcoholism.
In this presentation we will also try to point out those features that justify to consider the Pfeffer method, or of the closed-duodenal-loop, as an experimental surgical model suitable to mimic the clinical condition of acute pancreatitis. Furthermore, we will analyze the properties of local anesthetics that, according to our contention, are valuable agents to either prevent and/or treat efficaciously an episode of biliary acute pancreatitis.
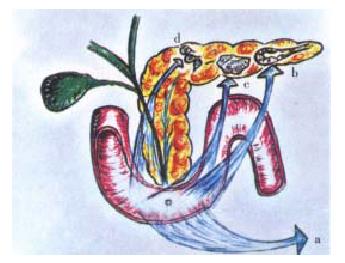
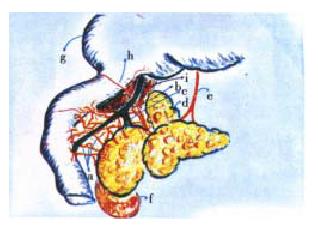
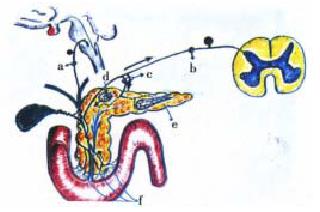
The clinical or surgical circumstances that usually evoke an episode of biliary acute pancreatitis, e.g. a stone that migrates into the duodenum or gets impacted in the distal end of the common bile duct[12,13], endoscopic maneuvers (sphincterotomy, sphinctero-manometry, retrograde cholangio-pancreatography), surgical manipulation, in or close to the duodeno-pancreas, percutaneous liver biopsy-associated hemobilia[14], have as a main starting locus of activation of the autonomic-arc-reflexes the peri-Vaterian duodenum. This intestinal segment is the most sensitive area of what we have conceived as the trigger of an imaginary pancreatic revolver (Figure 1). Our choice of this pedagogical reference was suggested, on the one hand, by the morphologic resemblance with the gun that the pancreatic gland offers in its general anatomical outline, and, on the other, by its functional characteristics[15,16]. The exquisite sensitivity of the trigger zone rests on its innervation density. This has been suggested, initially, by the macroscopic anatomical dissection findings in human cadavers and dogs[17-19]. Recently, by those observed in rats and the opossum (Figure 2). Subsequently, histochemical studies[20-23] and the utilization of very elaborate tracers[21-23], allowed to fully ratify the initial gross anatomical observations. Some of the nerve fibres that jump the duo deno-pancreatic cleft belong to the vagal system. They arrive to the head segment of the pancreas following a pathway through the gastric and duodenal walls [19,24-34]. Others contingents of nerve fibres are intrinsic to the duodeno-pancreas. They connect the enteric nervous system of the stomach and duodenum with the pancreatic gland (Figure 3). Some of them are cholinergic, and many are serotonergic. They end either in the pancreon units[15,16,18,19] and/or the intrapancreatic ganglia[17-19,20-23,25,26]. Following the same route, nerve fibres that belong to the afferent component of the autonomic nervous system jump the duodeno-pancreatic cleft in search of either the nodose ganglion of the vagus or the dorsal root ganglia of the spinal cord[35-46] . These duodeno-pancreatic nerve fibres are the basis of duodeno-pancreatic reflexes.
They are short, medium, and long[17-20,24-26,33]. The short duodeno-pancreatic reflex connect the enteric nervous system of the duodenum with the intrapancreatic ganglia and/or the bullets of the pancreatic revolver: the pancre on units and the Langerhansislets[21-23]. The medium duodeno-pancreatic reflex gets its integration in the celiac ganglia. The long duodeno-pancreatic reflex complete the arc reflex loop in the central nervous system, primarily in the hypothalamic-bulbar nuclei. A feature to emphasize is the presence of command neurons in the peri-Vaterian duodenum[28-32]. They exert a pre-programmed control of the interdigestive motor complex. Concerning the source of the duodenal innervation, its proximal segment, at least in the rat, is under the control of the left or anterior vagal nerve. Contrariwise, the peri-Vaterian duodenum is primarily subjected to nervous influences coming from the celiac ganglia[47-61].
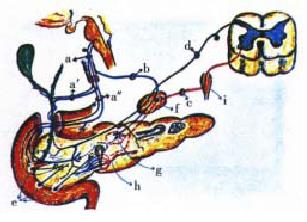
The pancreatic gland innervation depends on the following complex systems: the vagal, the splanchnic-celiac, the entero- pancreatic and the sensory-afferent[17-26,40-46], (Figure 3). In the first two complexes, one should take into consideration the existence of three different kinds of impulses: cholinergic, adrenergic and peptidergic. The above description is valid for both the exocrine and the endocrine pancreas[46]. Summarily, the vagal complex arrives to the pancreatic gland following the pathways of either the digestive tract wall (stomach-duodenum) or through the celiac ganglia. Its nerve fibres are essentially afferent. It also carries peptidergic influences and efferent impulses, primarily cholinergic. The splanchnic-celiac-ganglia-complex is also a nervous afferent pathway, namely of the pain sensation. From the efferent point of view, this complex is primarily of sympathetic impulses. Secondarily, is a transmitter of peptidergic and cholinergic efferent impulses. The enteropancreatic-complex is the result of neurons that project from the gut to the pancreas. According to the Gershon group[21-23], the nerve cells are essentially cholinergic and serotonergic. They influence the activity of intrapancreatic neurons and islet cells[46]. The sensory-afferent of the autonomic nervous system, through the mediation of its fine, amyelinic, capsaicin-sensitive type C fibres, constitute the basis of the neurogenic-inflammation[35-39,59-65].
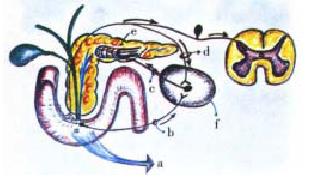
As a preamble to the consideration of the autonomic-arc-reflexes, it seems app ropriate to point out that the autonomic ganglia in the extrahepatic bile ducts and the pancreatic gland display similar histoarchitectural and physiologic features to those of the enteric nervous system. This is explainable by their common embryological origin[25,26]. When deprived of all outside neurons influences, the intrapancreatic ganglia can be shown to possess an intrinsic activity. The latter, coupled to outside cholinergic, sympathetic and peptidergic impulses give origin, within the gland, to what has been described, with tacit consensus, as cholinergic tone[15-18,25-27]. This is involved in an intricate interaction with the hormonal system. It has been ascertained on both pancreon segments: the acinar and the centroacinar-ductal, responsible, respectively, of the ecbolic and the hydrelatic components of the exocrine pancreatic secretion[15,16]. A feature to emphasize is the role of the celiac ganglia as an integration center of different kinds of autonomic arc reflexes[17-19,21-25,47-61]. The nerve fibres that arrive at the celiac ganglia come from different segments of the digestive tract, the extrahepatic bile ducts and the pancreas. In this autonomic nervous structure they establish an intricate interrelationship. The Faradic electrical stimulation of the celiac ganglia triggers an intrapancreatic ischemic process. This evolves as a result of the opening of arterio-venous shunts[50-57]. We have delineated this autonomic-arc-reflex as sympatho-ischemic (Figure 4). It has been clearly observed subsequent to the instillation of bile or bile salts in the pancreatic ductal tree[53]. The changes in the pancreatic vessels (spasms) and the gland’s capillary circulation (ischemia) have been objectivized in plastic casts[53]. The full development of the sympatho-ischemic autonomicarc-reflex leads to a depression of the pancreon’s secretory process. The exocrine pancreatic secretion inhibition can also be evoked through another type of autonomic-arc-reflex. We have pinpointed it as secretory-inhibitor. Its conception arose from our observation in canines equipped with duodenal Thomas can nula and studied in the conscious state (Pavlov frame). Indeed, when intemperate maneuvers are employed, especially if either liquid or air is injected abruptly into the main pancreatic duct (distention), a sudden arrest of exocrine pancreatics ecretion is frequently observed. This secretory-inhibitor autonomic-arc-reflex can be elicited from other segments of the digestive tract. We have observed it in rats when distending with a balloon either the antral-fundic junction of the stomach or the peri-Vaterian duodenum segment. A nervous reflex of this sort has been described by Warkentin et al[49] on bile secretion when di stending the colon or its nerve supply.
The pathway of this autonomic-arc-reflex is probably that of the afferent com ponent of autonomic nervous system. At pancreas level, an antidromic mechanism (pseudo-axonic), and/or an arc reflex loop integrated either in the intrapancreatic or the celiac ganglia is a possibility. The involvement of a peptide like CGRP[66], PP[67] or some other agent, like serotonin could be a logical assumption to make. The intimate physiopathogenic mechanism is probably related to with the recent studies of Ohshio et al[6] in rats. According to these authors, a short-term pancreatic duct obstruction interferes with the acinar cell secretory process down-stream of hormone-receptor binding, intracellular Ca2+ release and protein-kinase activation. The pseudo-axonic autonomic-arc-reflex, that leads to neurogenic inflammation, is evoked as a result of the irritation of the afferent component of the autonomic nervous system Normally, through these nerve fibres a significant percentage of the information originated in the stomach, duodenum, extrahepatic bile ducts and pancreas reach the central nervous system. The impulses travel through special type of nerve fibres: thin, unmyelinated, capsaicin-sensitive (type C). In their course to the nodose ganglion of the vagus, or the dorsal roots ganglia of the spinal cord, they send collaterals where they exert a modullatory influence on the neural transmission (Figure 3 and Figure 5). It is interesting that capsaicin (red-pepper agent), infused intraduodenally, evokes an exocrine pancreatic secretion equivalent to a 15% of that obtained with a maximal dose of CCK[63]. This pancreon’s secre tory response results from the activation of CCK-A receptors by the peptide CGRP released from the afferent nerve fibres. Others vasoactive peptides are involved, like SP, neurokinin-A, VIP, SOM and DYN. After synthesis in the somata of the afferent neurons, the bulk of the peptides is transported to their peripheral endings. The co-released peptides interact in the control of pancreatic function. Their antidromic release elicit vasodilatation and extravasation of plasma proteins and formation of edema (neurogenic inflammation)[35-39,59-65,69-71]. A feature to take into account for its pathophysiologic implications is that SP, and other sensory peptides released in the pseudo-axonic reflex, are capable of activating mast-cells to release histamine and other factors. Furth ermore, leukocytes, particularly neutrophils granulocytes, monocytes and lymphocytes are stimulated to adhere to the vascular endothelium and to emigrate to the surrounding tissue. Once neurogenic inflammation is triggered, monocytes release prostaglandins, thromboxane and cytokines[36-39]. Some of the latter agents are vasoactive by themselves and, in addition, can activate afferent nerve endings and thereby provide a positive feedback loop which reinforces the initial stimulation of the afferent component of the autonomic nervous system (Figure 3 and Figure 5). This whole process probably favors the absorption of endotoxin from the gut lumen into the blood stream. This agent is capable of inducing pancreatic lesions, depression of the exocrine pancreatic secretion and, in the liver, on the one hand, inhibition of albumin secretion, and, on the other, enhancing the secretion of both fibrinogen and C-reactive protein[72]. In the afferent component of the autonomic nervous system it should be pointed out the presence of different types of receptors: chemoreceptors, chemonociceptors, polymodal nociceptors and warmth receptors[60]. These nervous structures allow to detect those noxious stimuli that are potentially or actually harmful to the tissues. Besides, they also sense innocuous physiologic stimuli, such as pH, bile, distention. The afferent neurons represent a first line of defense against trauma. or, as in the stomach, to the injurious effects of ethanol[62,63]. It is probable that the same happens in the pancreatic gland. That, normally, in this organ, the afferent component of the autonomic nervous system fulfill some sort of cytoprotective function against the deleterious effects of injurious agents, can be inferred by the fact that its permanent ablation, like the one that results from long-term surgical bilateral splanchnicectomy, aggravates the pancreatic lesions (necrosis) induced by 24 h closed-duodenal-loop. This at least has been our experience in unpublished observations in rats[73] .
All the foregoing support the notion of autonomic-arc-reflexes involvement in the physiopathogenesis of biliary acute pancreatitis. Their degree of participation surely varies in each clinical case. This depending on the intrinsic characteristics of the injurious agent, its degree of persistence and the patient’s neuroendocrine reactivity.
When bile and/or pancreatic juice cannot reach the duodenal lumen, the entero-pancreatic feedback loop gets interrupted. This triggers the rising of blood’s CCK levels, and, through the induction of an increased cytosolic Ca2+ con centration, a supramaximal stimulation of the pancreon’s acinar cells is elicited[74-90]. The foregoing is linked to the evoking of both positive and negative duodeno-pancreatic reflexes[74]. Indeed, when in the duodenal lumen the influence normally exerted by bile, trypsin and chymotrypsin lessens (bile and/or pancreatic juice diversion bile and/or pancreatic duct obstruction) the releasing of CCK from the duodenal mucosa is markedly enhanced. This peptide through a paracrinic mechanism in the duodenal wall, activates the autonomic nervous system. Thus, a positive duodeno-pancreatic-reflex (increased intrapan creatic cholinergic tone) is induced. This effect is potentiated by a concomit ant annulment of a negative duodeno-pancreatic- reflex. The latter was postulated by us in the eighties[74]. It might have as a pathway the entero-pan creatic nervous complex. A neurotransmitter (SOM 问号, PP 问号, serotonin 问号) induced by the presence in the duodenal lumen of trypsin, chymotrypsin and/or bile, might be at the basis of restricting influences on the pancreon units (Figure 6).
Others features to take into account to better understand the entero-pancreatic feedback loop is that a monitor peptide in the pancreatic juice, and a CCK-releasing peptide, derived from the duodenal mucosa, exert a direct stimulating action on the CCK-releasing cells (I cells). These small peptides are normally in activated by intraduodenal trypsin and chymotrypsin. Concerning bile, this secretion, besides activating the postulated negative duodeno-pancreatic-reflex, normally exerts an indirect feedback inhibition. The latter might result from the stabilization of pancreatic juice’s protease by the calciumions in it contained[74,83-90]. A physiologic detail to emphasize is that unblocking of the bile obstruction allows the duodenal mucosa to release secretin[91,92]. This hormone may help to dislodge, through an enhanced exocrine pancreatic secretion of water and bicarbonate, eventual intrapancreatic protein plugs. Beside s, it may attenuate the noxious influences of CCK on the pancreon’s acinar cells. The latter has been shown, initially by Kanno et al[93], subsequently by Renner et al[94], and, recently, through an agent (tetrafrenyl actone) that has proved to possess a releasing capacity of this hormone from the intestine[95]. A feature to be emphasized is that both secretin and VI P, through a second messenger (cAMP), either prevent or restore, in the acinar cells, the CCK-induced disrupted microtubules and microfilaments.
When the usual agents that elicit the biliary acute pancreatitis exert their actions superimposed on a background of chronic alcoholism, the degree and extensi on of the pancreatic acute inflammatory lesions are significantly enhanced[96,97]. This notion is important because the incidence of a chronic ethanol intoxication as a background of an episode of biliary acute pancreatitis is rampant. This assertion is confirmed by several recent papers[96,97]. Experimentally, Gronros et al[98-100] have ratified, in rats, our primary contention, described in dogs and rats[101-118], that in the pathophysiology of ethanol-evoked pancreatic lesions two major factors play a crucial role: an elevated intrapancreatic acetylcholine level (high cholinergic tone) and an enhanced acinar cell response to CCK. At clinical level, Brugge et al[119] have provided additional support to this postulation. Recently, we have suggested[115,117,118] that the forementioned changes might be consequence of an alcohol-induced loss of a normal braking mechanism exerted by higher autonomic nervous centers. The braking on the pancreon units might depend on peptides like CGRP[66], PP[67] and/or SOM[120,121].
As eventual aggravating influences associates to alcohol intoxication, one should consider the probable participation of an ethanol-evoked sphincter of Oddi dysfunction. Two reports give support to this assumption. The first one, performed in monkeys[128], has disclosed that after the administration of a 130 mL/L ethanol solution there is a significant increase of the main pancreatic pressure. The second report[121], has put in evidence, in humans, that acute intraduodenal ethanol induces an increase of the Vaterian resistance. This phenomenon is enhanced when performed in chronic alcoholics.
Others additional effects related to ethanol intoxication that merit their consideration are: the reduction of the capillary blood flow[122] and the activation and migration of leukocytes in the pancreatic gland[123].
At this stage, it seems relevant to analyze the data afforded by the experimental, surgical-induced acute pancreatitis of the Pfeffer method or of the closed-duodenal-loop[124-131]. This procedure, according to our view, offers a suggestive approximation to those conditions which usually interplay, in a clinical setting, in an episode of biliary acute pancreatitis. Indeed, the closed- duodenal-loop model offers a series of conditions that somehow mimick those frequently seen in human cases of biliary acute pancreatitis, e.g. the distention and chemical injury of the peri-Vaterian duodenum, the bile-pancreatic hypertension, the eventual reflux of the duodenal content into the bile-pancreatic ducts, the disruption of the entero-pancreatic feedback loop due to the exclusion of both bile and pancreatic juice from the intestinal lumen and the bacterial aggression (endotoxemia).
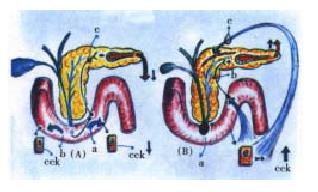
In recent experiments in rats with a short-term closed-duodenal-loop method, that we have modified from Orda et al[132] and De Rai et al[133], we have observed changes of the pancreatic gland that, taking into account the brevity of the experiments, were unexpected[134]. Indeed, after filling a duodenal loop with a 70 g/L taurocholate solution plus a few drops of blue-methylene, under a constant pressure of a 110 cm fluid column height, and keeping those conditions for only 3 min, the macroscopic evaluation of the pancreatic gland disclosed, 3 h after, the presence of edema and spotty foci of acinar cell necrosis (Figure 7). Remarkably, the above changes were obtained under the total absence of any reflux of the duodenal content into the bile-pancreatic ducts. This crucial detail was verified by means of a constant visual observation of the duodeno-pancreas. Something we learned from the above test, is that it takes normally more than 20 min, and sometimes even more than an hour, to appreciate the reflux of the duodenal content into the bile-pancreatic ducts. When this does indeed occur, a marked pancreatic edema is seen, and quite rapidly, a notorious hemorrhagic acute pancreatitis distinctly develops.
The above described results give further solid support to our contention that the irritation of the duodenum, at the level of the peri-Vaterian duodenum (trigger zone of the pancreatic revolver) is capable of activating autonomic-arc-reflexes. The latter, coupled with the changes evoked by the disruption of the entero-pancreatic feedback loop (bile-pancreatic obstruction) (Figure 6), probably explains, as we have already pointed out, the physiopathogenesis of the pancreatic lesions. This speculations of ours, that biliary acute pancreatit is might pivot around the activation of autonomic-arc-reflexes and a disruption of the entero-pancreatic feedback loop could perhaps be extended to explain, at least partially, the liver changes that recently have been pointed out by Isogai et al[135] in human patients with biliary acute pancreatitis. Indeed, in the reports of these authors, a feature to be emphasized is that both the liver’s histopathologic changes and the biochemical abnormalities were of the same order of magnitude in patients with and without impacted stones in the Vaterian region. This set of circumstances allow to infer that besides bile duct hypertension other factors (autonomic reflexes) might be at the basis of the above findings. An anatomical detail that gives support to the precedent contention is that of the rich density of nerve fibres in the hepatic hilum that we have put in evidence in macroscopic dissection studies in human cadavers[17-19].
Another observation that deserves to be emphasized because it affords additional indirect basis to presume an involvement of autonomic-arc-reflexes in the physiopathogenesis of biliary acute pancreatitis is the one associated to a pure distention of bile ducts without the intervention of any intemperate maneuver or the irritation of a chemical agent. This is what suggestively happens with the acute pancreatic inflammation that have been reported in cases of hemobilia, accidental or iatrogenic (post-percutaneous liver biopsy)[14].
All the above findings give coherent basis to consider an eventual beneficial effect of local anesthetics either as preventive and/or therapeutic agents of an episode of biliary acute pancreatitis. A long experience in conscious dogs with lidocaine spray in the peri- Vaterian duodenum[19,24,28,30,33] has convinced us, on the one hand, of their efficacy to interrupt noxious autonomic-arc-reflexes and depress the intrapancreatic cholinergic tone, and, on the other, of their relaxing capacity on both the main pancreatic duct outlet and the duodenal motor activity. The above anesthetic-induced changes make easier the catheterization of the Vater papilla in man. Consequently, the changes of its traumatization are significantly reduced. A detail to be emphasized is that of the atropine-like effects evoked by local anesthetics (procaine, lidocaine) in the duodenum and the sphincter of Oddi described by Varela-López et al[136,137], by Velasco Surez[138,139], Cottini[24] and by our group[24,28,30,33].
The forementioned authors have given convincing evidence of the local anesthetic value no treating clinical cases of sphincter of Oddi dysfunction or bouts of recurrent stone impaction in the distal common bile duct. The procedure most frequently used was of a duodenal infusion of procaine clorhidrate (20 mL of a 10 g/L solution) or an oral ingestion of this anesthetic (0.2 g up to 1.2 g/24 h). With this latter approach, we succeeded in sparing a sphincterotomy in several patients with common bile duct residual stones following a laparoscopic cholecystectomy.
It is worth remembering that Albanese[69], Longo and Sosa Gallardo[64,65], Salazar[59] and Ochsner[70] have given solid accounts of the clinical therapeutic value of a temporary interruption of the autonomic -arc-reflexes by means of a local anesthetic of the celiac ganglia. This was experimentally ratified by the group of Waisman[58]. Indeed, these authors have shown, in rats subjected to a 24 h closed-duodenal-loop procedure, that those animals in which a single infiltration with lidocaine was performed had a significantly longer survival than the controls.
Local anesthetic bathing of the duodenum, namely at the level of the peri-Vaterian duodenum, or the anesthetic infiltration of the duodeno-pancreatic cleft, and/or of the celiac plexus, during an eventual laparotomy, constitute, according to our postulation, an efficacious means to prevent, or attenuate, the intensity of autonomic-arc-reflexes. Furthermore, they surely contribute to depress the intrapancreatic cholinergic tone. This is important in cases of an episode of biliary acute pancreatitis superimposed on a background of chronic alcoholism. They might do this through the interruption, on the one hand, of cholinergic impulses that course through the gastro duodenal wall, and, on the other, by blocking the CCK release from the “I” endocrine cells and the subsequent evoking of duodeno-pancreatic reflexes[18,19,25,26,63,140]. The latter is suggestively supported by the recent demonstration that vagal mucosal receptors are directly sensitive to CCK-8 [141,142]. Besides, as it has been pointed out by Bj-rck et al[71] and Mc Cafferty et al[143], many other properties of local anesthetics, in addition to those of inhibiting action potentials by blocking sodium channels, are surely involved. The foregoing might explain the therapeutic success reported with intrarectally infused lidocaine in the treatment of ulcerative colitis[71]. In a recent report[144], it has been shown that in patients subjected to ERCP and therapeutic endoscopy (e.g. sphincterotomy, stone removal, etc.) and randomly assigned to have ① 10 mL of 10 g/L lidocaine sprayed onto the ampulla before or after, or, ② saline, either pre or post ERCP, that local anesthesia applied to the ampulla before ERCP facilitate sc annulation of the ampulla and appears to reduce hyperamylasemia whether given before or after ERCP. When considering further experimental evaluation with local anesthetics, related to either the prevention or treatment of biliary acute pancreatitis, one should take into consideration the results recently reported in rats by Mc Cafferty et al[143]. Indeed, it is remarkable that the intrarectal administration of 0.5 mL of 25 g/L carboxymethylcellulose, containing lidocaine hydrochloride, at dosis ranging from 5 mg/kg to 100 mg/kg had induced a significative reduction of the colitic score and of the myeloperoxidase activity.
In recent experiments in rats with our “shortterm-closed-duodenal-loop” model[134], we showed that previous bathing of the duodenum with lidocaine (20 mg/kg) reduced the histopathologic score of pancreatic necrosis observed in the control group. This was confirmed in a subsequent series[145]. It is our postulation that through the interruption of autonomic-arc-re flexes one could interfere in the release of cytokines at pancreas level[146-154]. This is also suggested by our recent findings in the opossum[155].
Concerning the variable effects of a local anesthetic (lidocaine) on exocrine pancreatic secretion according to the route of administration, e.g. intraduodenal vs intravenous, we have acquired a long experience in dogs equipped with a duodenal fistula (Thomas cannula) and tested in the conscious state[33]. From them, we have learned that spraying of the papillar zone (trigger of the pancreatic revolver) with lidocaine (50 mg each, 10 min for 2 h) induces a significant depression (60%) of the plateau levels of all secretin-induced parameters. Remarkably, and coherent with the anatomical details previously outlined, the above exocrine pancreatic secretion changes were not observed with lidocaine spraying outside the papillar zone. Other suggestive findings were, firstly, that the intravenous infusion of the same amount of lidocaine (500 mg dissolved in 200 mL physiological saline = 12.5 mg/kg), did not modify any of the exocrine pancreatic secretion parameters and secondly, that when the intraduodenal lidocaine testing was performed in alcohol-fed dogs (2-year), the degree of the anesthetic-induced depression of the exocrine pancreatic secretion was less notorious (35%) than when carried out in controls (65%). We have interpreted this difference as a reflection in the ethanol- fed animals of a higher duodeno-pancreatic cholinergic tone. It is interesting that at clinical level this assertion has been ratified by Brugge et al[119]. Other considerations that seem relevant to point out is that of the extran euronal effects of local anesthetics. In addition to our presumption that through the interruption of autonomic-arc-reflexes they interfere with the release of inflammatory mediators at pancreas level, they inhibit the phospholipase A2 enzyme and its interaction with its specific substrate: the cell membrane phospholipids. The latter was shown by Aho et al[151] following their experiments on acute pancreatitis treated with a procaine solution (40 mg/kg).
Finally, and in order to complete the attempt to interrupt the evolving of the mechanism that we have considered as pivotal in the physiopathogenesis of biliary acute pancreatitis, it would seem logical to add to local anesthetics the simultaneous administration of pancreatic enzymes[75,83,86,87] and, eventually, of a calcium channel blocker[89,152]. Concerning the former, the oral and/or the intragastric-intraduodenal administration of pancreatic enzymes, might accomplish, on the one hand, the depression of the CCK release from the intestinal mucosa, and, on the other, the evoking of our previously described neural mechanism of “pancreon” inhibition, the negative duodeno-pancreatic reflex. In relation with calcium channel blockade, it is interesting to point out the recent finding of Hughes et al[153] in rats. Indeed, these authors have shown that the administration of diltiazem is assocated with significant reduction in serum TNF-alfa levels as well as amelioration of pan creatitis by biochemical and pathological criteria. They emphasize that TNF-alfa mediates tissue injury through the activation of inflammatory cells, the up regulation of adhesio nmolecules, the production of nitric oxide and the release of other cytokines and mediators of inflammation.
Edited by Pan BR
Proofread by Ma JY
| 1. | Berk JE. Bockus Lecture: World Congress of Gastroenterology, Los Angeles, CA--October 3, 1994. The management of acute pancreatitis: a critical assessment as Dr. Bockus would have wished. Am J Gastroenterol. 1995;90:696-703. [PubMed] |
| 2. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 597] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 3. | Lerch MM, Hernández CA, Adler G. Acute pancreatitis. N Engl J Med. 1994;331:948-949. [PubMed] |
| 4. | Uomo G, Rabitti PG, Laccetti M, Visconti M. Pancreatico-choledochal junction and pancreatic duct system morphology in acute biliary pancreatitis. A prospective study with early ERCP. Int J Pancreatol. 1993;13:187-191. [PubMed] |
| 5. | Lerch MM, Saluja AK, Dawra R, Ramaraò P, Saluja M, Steer ML. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992;103:205-213. [PubMed] |
| 6. | Lerch MM, Saluja AK, Rünzi M, Dawra R, Saluja M, Steer ML. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993;104:853-861. [PubMed] |
| 7. | Rünzi M, Saluja A, Lerch MM, Dawra R, Nishino H, Steer ML. Early ductal decompression prevents the progression of biliary pancreatitis: an experimental study in the opossum. Gastroenterology. 1993;105:157-164. [PubMed] |
| 8. | Weiner GR, Geenen JE, Hogan WJ, Catalano MF. Use of corticosteroids in the prevention of post-ERCP pancreatitis. Gastrointest Endosc. 1995;42:579-583. [PubMed] |
| 9. | Sternberg EM. Neural-immune interactions in health and disease. J Clin Invest. 1997;100:2641-2647. [PubMed] |
| 10. | Chen YK, Abdulian JD, Escalante-Glorsky S, Youssef AI, Foliente RL, Collen MJ. Clinical outcome of post-ERCP pancreatitis: relationship to history of previous pancreatitis. Am J Gastroenterol. 1995;90:2120-2123. [PubMed] |
| 11. | Khodkov K, Siech M, Beger HG. Cyst of the common bile duct in combination with pancreas divisum as a cause of acute pancreatitis. Pancreas. 1996;12:105-107. [PubMed] |
| 12. | Opie EL. The relation of cholelithiasis to disease of the pancreas and to fat necrosis. Am J Med Sci. 1901;121:27-43. |
| 13. | Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484-487. [PubMed] |
| 14. | Van Os EC, Petersen BT. Pancreatitis secondary to percutaneous liver biopsy-associated hemobilia. Am J Gastroenterol. 1996;91:577-580. [PubMed] |
| 15. | Tiscornia OM. Concepto de Pancreon. In: Pérez V, de Larrechea I, Arabethy J, Tiscornia OM, editors. Gastroenterologia. El Ateneo 1971; 470-484. |
| 16. | Dreiling D, Tiscornia OM. Tests of pancreatic function. Scientific foundation of gastroenterology. Londres: W. Heinemann Medical Book 1980; 591-601. |
| 17. | Tiscornia OM, Martinez JL, Sarles H. Some aspects of human and canine macroscopic pancreas innervation. Am J Gastroenterol. 1976;66:353-361. [PubMed] |
| 18. | Tiscornia OM. Contrle nerveux cholinergique du pancréas. Biol Gastroenterol (. Paris). 1976;9:255-270. |
| 19. | Tiscornia OM. The neural control of exocrine and endocrine pancreas. Am J Gastroenterol. 1977;67:541-560. [PubMed] |
| 20. | Anglade P, Michel C, Rozé C. Intrinsic nerves of the pancreas after celiac and superior mesenteric ganglionectomy in rats: a morphologic study of acetylcholinesterase activity and catecholamine histofluorescence. Pancreas. 1987;2:568-577. [PubMed] |
| 21. | Kirchgessner AL, Gershon MD. Innervation of the pancreas by neurons in the gut. J Neurosci. 1990;10:1626-1642. [PubMed] |
| 22. | Kirchgessner AL, Mawe GM, Gershon MD. Evaluation of the activity of chemically identified enteric neurons through the histochemical demonstration of cytochrome oxidase. J Comp Neurol. 1990;301:1-14. [PubMed] |
| 23. | Kirchgessner AL, Gershon MD. Presynaptic inhibition by serotonin of nerve-mediated secretion of pancreatic amylase. Am J Physiol. 1995;268:G339-G345. [PubMed] |
| 24. | Tiscornia OM. Importancia de la región Vateriana en la patología bilio-pancre tica. Puestaen evidencia de reflejos duodeno pancre ticos. Rev Argent Cirug. 1979;36:232-239. |
| 25. | Tiscornia OM, Dreiling DA, Yacomotti J, Kurtzbart R, de La Torre A, Farache S. Neural control of the exocrine pancreas: an analysis of the cholinergic, adrenergic, and peptidergic pathways and their positive and negative components. 1: Neural mechanisms. Mt Sinai J Med. 1987;54:366-383. [PubMed] |
| 26. | Tiscornia OM, Dreiling DA, Yacomotti J, Kurtzbart R, de la Torre A, Farache S. Neural control of the exocrine pancreas: an analysis of the cholinergic, adrenergic, and peptidergic pathways and their positive and negative components. 2. Integration of neural and hormonal mechanisms. Mt Sinai J Med. 1988;55:126-131. [PubMed] |
| 27. | Tiscornia OM, Cresta MA, Negri G, Lehmann ES de, Vaccaro MI, Resnik R, Celener D, Hamamura S, Mora MI, Bustos Fernndez L. Sistema nervioso autónomoy pncreas exocrine endocrino en la rata. Acta Gastroenterol Latino Amer. 1991;21:204. |
| 28. | Tiscormia OM, Cresta MA, Celener D, Hamamura S, De Paula J, Celener P, Farache S, Negri G. Centro neural peri-Vateriano en la rata. Evidencias indirectas brindadas porla exclusión Vateriana, la anestesia papilar, lasolarectomíay la vaguectomía troncular bilateral distal. Acta Gastroenterol LatinoAmer. 1991;21:204. |
| 29. | Tiscornia OM, Hamamura S, Celener D, Cresta MA, Negri G, Gonz-lez E, Lehmann ES de, Tiscornia-Wasserman PG. An overview of gastro-duodeno-pancreas innervation in the rat. Emphasis on some disregarded anatomical structures (Abstr). Am J Gastroenterol. 1993;88:1544. |
| 30. | Tiscornia OM, Hamamura S, Cresta MA, Lehmann ES de, Celener D, Negri G, Gonzlez E, Tiscornia Wasserman PG. Duodenal peri-Vaterian autonomic nervous center in the rat: Indirect evidences give by Vaterian papillary anesthesia, Vaterian exclusion, supra and infra Vaterian transection and reanastomosis, celiac ganglionectomy and distal bilateral truncal vagotomy. Am J Gastroenterol. 1993;88:1565. |
| 31. | Tiscornia OM, Hamamura S, Celener D, Gonzalez E, Cresta MA, Vaccaro MI, Negri G, Lehmann ES de, Cerini C, Waisman H. Caracterización antomo-histo-trófico-funcional de dos centros autonómicos periféricos: el de launión antro fúndicayel peri Vateriano en la rata. Rev del Hospital de Clínicas de Bs As, 1992; 6: 29. Acta Gastroenterol Latino Amer. 1993;23:56. |
| 32. | Tiscornia OM, Tiscornia Wasserman PG, Hamamura S, Cresta MA, Negri G, Lehmann ES de, De Paula J, Yacomotti J, Farache S. Síntesis conceptual de la inervación macroscópica gastro duodeno pancre tica. Revisión centrada en una investigación anatómica en la rata. Rev del Hospital de Clínicas de Bs As, 1992; 6: 28-29. Acta Gastroenterol Latino Amer. 1993;23:57. |
| 33. | Tiscornia OM, Sarles H, Voirol M. Evidences for duodeno pancreatic reflexes and an anti CCK factor with lidocaine infused intravenously and sprayed topically in pancreatic papilla in nonalcoholic and alcohol fed dogs. Am J Gastroenterol. 1976;66:221-240. |
| 34. | Tiscornia OM, Celener D, Cresta MA, Perec CJ, Tumilasci O, Dreiling DA. Trophic and antitrophic circuits controlling pancreatic weight in the rat. Mt Sinai J Med. 1986;53:343-355. [PubMed] |
| 35. | Holzer P. Peptidergic sensory neurons in the control of vascular functions: mechanisms and significance in the cutaneous and splanchnic vascular beds. Rev Physiol Biochem Pharmacol. 1992;121:49-146. [PubMed] |
| 36. | J-ning W. Pain and the sympathetic nervous system. Pathophysiologic mechanisms. Autonomic failure. Oxford: Oxford University Press 1992; 231-251. |
| 37. | Kowalski ML, Kaliner MA. Neurogenic inflammation, vascular permeability, and mast cells. J Immunol. 1988;140:3905-3911. [PubMed] |
| 38. | Payan DG. Substance P: a modulator of neuroendocrine-immune function. Hosp Pract (. Off Ed). 1989;24:67-73, 76, 78-80. [PubMed] |
| 39. | Kiernan J. Interactions between mast cells and nerves. Neurogenic inflammation. Trends Pharmacol Sci. 1990;11:316. [PubMed] |
| 40. | Li Y, Owyang C. Vagal afferent pathway mediates physiological action of cholecystokinin on pancreatic enzyme secretion. J Clin Invest. 1993;92:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Lu Y, Owyang C. Secretin at physiological doses inhibits gastric motility via a vagal afferent pathway. Am J Physiol. 1995;268:G1012-G1016. [PubMed] |
| 42. | Sánchez-Vicente C, Rodríguez-Nodal F, Minguela A, García LJ, San Román JI, Calvo JJ, López MA. Cholinergic pathways are involved in secretin and VIP release and the exocrine pancreatic response after intraduodenally perfused acetic and lactic acids in the rat. Pancreas. 1995;10:93-99. [PubMed] |
| 43. | Li P, Chang TM, Chey WY. Neuronal regulation of the release and action of secretin-releasing peptide and secretin. Am J Physiol. 1995;269:G305-G312. [PubMed] |
| 44. | Bockman DE. Toward understanding pancreatic disease: from architecture to cell signaling. Pancreas. 1995;11:324-329. [PubMed] |
| 45. | Adler G, Nelson DK, Katschinski M, Beglinger C. Neurohormonal control of human pancreatic exocrine secretion. Pancreas. 1995;10:1-13. [PubMed] |
| 46. | Brunicardi FC, Shavelle DM, Andersen DK. Neural regulation of the endocrine pancreas. Int J Pancreatol. 1995;18:177-195. [PubMed] |
| 47. | Popielski L. Zur physiologic des plexus coeliacus. Arch F Anatu Physiol. Oxford: Oxford University Press 1903; 338-360. |
| 48. | Kuntz A, Van Buskirk C. Reflex inhibition of bile flow and intestinal motility mediated through decentralized celiac plexus. Proc Soc Exp Biol Med. 1941;46:519-523. |
| 49. | Warkentin J, Huston JH, Puestow FW, Ivy AC. The mechanism of bile flow inhibition upon distention of the colon or stimulation of its nerve supply. Am J Physiol. 1943;133:462-464. |
| 50. | KUNTZ A, RICHINS CA. Effects of direct and reflex nerve stimulation on the exocrine secretory activity of pancreas. J Neurophysiol. 1949;12:29-35. [PubMed] |
| 51. | RICHINS CA. Effect of sympathetic nerve stimulation on blood flow and secretion in the pancreas of the cat. Am J Physiol. 1953;173:467-470. [PubMed] |
| 52. | Gilsdorf RB, Urdalena T, Delaney JP, Leonard AJ. Central nervous system influences on pancreatic secretion, sphincteric mechanism and blood flow and their role in the effect of bile induced pancreatitis. Surgery. 1967;62:581-588. |
| 53. | Papp M, Ungvari G, Németh PE, Munkacsi I, Zubek L. The effect of bile-induced pancreatitis on the intrapancreatic vascular pattern in dogs. Scand J Gastroenterol. 1969;4:681-689. [PubMed] |
| 54. | Varga B, Folly G, Papp M. L' effet de I’excitation eléctrique du ganglion coeliaque sur le débit sanguin du pancréas. Lyon Chirugical. 1974;70:168-170. |
| 55. | Szurszewski JH. Toward a new view of prevertebral ganglion. Nerves and the gut. New York: Slack 1997; 224-260. |
| 56. | Kreulen DL, Szurszewski JH. Reflex pathways in the abdominal prevertebral ganglia: evidence for a colo-colonic inhibitory reflex. J Physiol. 1979;295:21-32. [PubMed] |
| 57. | Kreulen DL, Muir TC, Szurszewski JH. Peripheral sympathetic pathways to gastroduodenal region of the guinea pig. Am J Physiol. 1983;245:G369-G375. [PubMed] |
| 58. | Martin S, Ameri C, Waisman H. Pancreatitis aguda experimental en la rata. Acción de la lidocaina instilada en el plexo solar. Rev Arg Cirug. 1985;48:126-128. |
| 59. | Salazar JR. In: C tedra de Cirugia Facultad de Ciencias Médicas de la Universidad de Córdoba. Pancreatitis Aguda, editor. Córdoba Argentina. . |
| 60. | Cervero F, Sharkey KA. An electrophysiological and anatomical study of intestinal afferent fibres in the rat. J Physiol. 1988;401:381-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | De Giorgio R, Sternini C, Brecha NC, Widdison AL, Karanjia ND, Reber HA, Go VL. Patterns of innervation of vasoactive intestinal polypeptide, neuropeptide Y, and gastrin-releasing peptide immunoreactive nerves in the feline pancreas. Pancreas. 1992;7:376-384. [PubMed] |
| 62. | Barnes PJ, Belvisi MG, Rogers DF. Modulation of neurogenic inflammation: novel approaches to inflammatory disease. Trends Pharmacol Sci. 1990;11:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Gicquel N, Nagain C, Chariot J, Tsocas A, Levenez F, Corring T, Rozé C. Modulation of pancreatic secretion by capsaicin-sensitive sensory neurons in the rat. Pancreas. 1994;9:203-211. [PubMed] |
| 64. | Longo OF, Sosa Gallardo CA, Ferraris A. In: “Pancreatopatías Agudas: Estudio Patogénicoy Terapéutic”. Published by: Imprenta Universitaria de Córdoba. Rep Argentina 1954; . |
| 65. | Sosa Gallardo C, Kesner L, Ferraris A, Herrero A. Contribución clínico-experimental a la patogenia del infarto segmentario idiop tico del epiplónmayor. Bioly Trab Soc Arg de Cirug. 1960;21:5-28. |
| 66. | Li Y, Kolligs F, Owyang C. Mechanism of action of calcitonin gene-related peptide in inhibiting pancreatic enzyme secretion in rats. Gastroenterology. 1993;105:194-201. [PubMed] |
| 67. | Okumura T, Pappas TN, Taylor IL. Pancreatic polypeptide microinjection into the dorsal motor nucleus inhibits pancreatic secretion in rats. Gastroenterology. 1995;108:1517-1525. [PubMed] |
| 68. | Ohshio G, Okada N, Manabe T, Imamura M. Pancreatic exocrine secretion in short-term pancreatic duct obstruction induced acute pancreatitis in rats: an in vivo and in vitro study. Digestion. 1994;55:200-207. [PubMed] |
| 69. | Albanese AR, pataro V. Pancreatitis aguda. Su tratamiento por la anestesia del espl cnico. Prensa Médica Argentina. 1939;28:74-76. |
| 70. | DALE WA. Splanchnic block in the treatment of acute pancreatitis. Surgery. 1952;32:605-614. [PubMed] |
| 71. | Björck S, Dahlström A, Johansson L, Ahlman H. Treatment of the mucosa with local anaesthetics in ulcerative colitis. Agents Actions. 1992;Spec No:C60-C72. [PubMed] |
| 72. | Vaccaro MI, Dagrosa MA, Mora MI, Tiscornia OM, Sordelli DO. The effect of chronic intraperitoneal infusion of bacterial endotoxin on exocrine pancreas function in rats. Int J Pancreatol. 1996;19:49-54. [PubMed] |
| 73. | Stroff T, Plate S, Respondek M, Müller KM, Peskar BM. Protection by gastrin in the rat stomach involves afferent neurons, calcitonin gene-related peptide, and nitric oxide. Gastroenterology. 1995;109:89-97. [PubMed] |
| 74. | Tiscornia OM, Dreiling DA. Is basal bile-pancreatic secretion influenced by gastric juice diversion in the rat问号. Mt Sinai J Med. 1986;53:368-376. [PubMed] |
| 75. | Owyang C, Louie DS, Tatum D. Feedback regulation of pancreatic enzyme secretion. Suppression of cholecystokinin release by trypsin. J Clin Invest. 1986;77:2042-2047. [PubMed] |
| 76. | Burton FR, Burton MS, Garvin PJ, Joshi SN. Enteral pancreatic enzyme feedback inhibition of the exocrine secretion of the human transplanted pancreas. Transplantation. 1992;54:988-992. [PubMed] |
| 77. | Miyasaka K, Sazaki N, Funakoshi A, Matsumoto M, Kitani K. Two mechanisms of inhibition by bile on luminal feedback regulation of rat pancreas. Gastroenterology. 1993;104:1780-1785. [PubMed] |
| 78. | Murayama KM, Samuel I, Toriumi Y, Solomon TE, Turkelson CM, Joehl RJ. Increased circulating cholecystokinin in obstruction-induced acute pancreatitis. I. Bile duct obstruction with and without pancreatic duct obstruction. J Surg Res. 1993;54:126-131. [PubMed] |
| 79. | Toriumi Y, Samuel I, Wilcockson DP, Turkelson CM, Solomon TE, Joehl RJ. Increased circulating cholecystokinin in obstruction-induced acute pancreatitis. II. Pancreatic duct obstruction with and without bile duct obstruction. J Surg Res. 1993;54:132-135. [PubMed] |
| 80. | Samuel I, Toriumi Y, Wilcockson D, Joehl RJ. Pathogenesis of pancreatic duct obstruction induced acute pancreatitis in opossums is influenced by duodenal exclusion of pancreatic enzymes. Am J Surgery. 1993;165:742 (A). [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 81. | Kim CD, Lee KY, Chang TM, Chey WY. Negative feedback regulation of pancreatic exocrine secretion in guinea pigs. Pancreas. 1995;10:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am J Physiol. 1995;269:G319-G327. [PubMed] |
| 83. | Mizutani S, Miyata M, Izukura M, Tanaka Y, Matsuda H. Role of bile and trypsin in the release of cholecystokinin in humans. Pancreas. 1995;10:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Miyasaka K, Funakoshi A. Involvement of gene expressions of cholecystokinin and secretin in luminal feedback regulation in conscious rats. Pancreas. 1995;10:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 85. | Funakoshi A, Miyasaka K, Jimi A, Nakamura E, Teraoka H. Changes in gene expression of pancreatitis-associated protein and pancreatic secretory trypsin inhibitors in experimental pancreatitis produced by pancreatic duct occlusion in rats: comparison with gene expression of cholecystokinin and secretin. Pancreas. 1995;11:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 86. | Spannagel AW, Green GM, Guan D, Liddle RA, Faull K, Reeve JR. Purification and characterization of a luminal cholecystokinin-releasing factor from rat intestinal secretion. Proc Natl Acad Sci USA. 1996;93:4415-4420. [PubMed] |
| 87. | Samuel I, Toriumi Y, Wilcockson DP, Turkelson CM, Solomon TE, Joehl RJ. Bile and pancreatic juice replacement ameliorates early ligation-induced acute pancreatitis in rats. Am J Surg. 1995;169:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Jungermann J, Lerch MM, Weidenbach H, Lutz MP, Krüger B, Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am J Physiol. 1995;268:G328-G338. [PubMed] |
| 89. | Zhou W, Shen F, Miller JE, Han Q, Olson MS. Evidence for altered cellular calcium in the pathogenetic mechanism of acute pancreatitis in rats. J Surg Res. 1996;60:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Samuel I, Joehl RJ. Bile-pancreatic juice replacement not cholinergic- and cholecystokinin-receptor blockade reverses acinar cell hyperstimulation after bile-pancreatic duct ligation. Am J Surg. 1996;171:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 91. | Osnes M, Hanssen LE, Lehnert P, Flaten O, Larsen S, Londong W, Otte M. Exocrine pancreatic secretion and immunoreactive secretin release after repeated intraduodenal infusions of bile in man. Scand J Gastroenterol. 1980;15:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Osnes M, Hanssen LE. The influence of intraduodenal administration of pancreatic juice on the bile-induced pancreatic secretion and immunoreactive secretin release in man. Scand J Gastroenterol. 1980;15:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Kanno T, Matsumoto T, Mort M, Oyamada M, Nevalainen T. Secretion prevents hyporeactive and morphological responses of rat pancreatic acinar cells to stimulation with supraoptimal concentration of cholecystokinin-octapeptide. Biomedical Research. 1984;5:355-370. |
| 94. | Renner IG, Wisner JR, Rinderknecht H. Protective effects of exogenous secretin on ceruletide-induced acute pancreatitis in the rat. J Clin Invest. 1983;72:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Tachibana I, Watanabe N, Shirohara H, Akiyama T, Nanano S, Otsuki M. Effects of tetraprenylacetone on pancreatic exocrine secretion and acute pancreatitis in two experimental models in rats. Int J Pancreatol. 1995;17:147-154. [PubMed] |
| 96. | Tiscornia OM. Pancreatitis Aguda. In: “Emergencias Médicasy Quirúrgicas”. Edited by Barè G, Bernabó J, Califano J and Waisman H. Published by EDIMED, Buenos Aries 1987; 276-303. |
| 97. | Foitzik T, Lewandrowski KB, Fernández-del Castillo C, Rattner DW, Klar E, Warshaw AL. Exocrine hyperstimulation but not pancreatic duct obstruction increases the susceptibility to alcohol-related pancreatic injury. Arch Surg. 1994;129:1081-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Grönroos JM, Kaila T, Aho HJ, Nevalainen TJ. Decrease in the number of muscarinic receptors in rat pancreas after chronic alcohol intake. Pharmacol Toxicol. 1989;64:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Grönroos JM. Pathogenesis of acute alcoholic pancreatitis. Lancet. 1990;335:1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 100. | Grönroos JM, Aho HJ, Nevalainen TJ. Effects of chronic alcohol intake and secretory stimulation on sodium taurocholate-induced pancreatic necrosis in the rat. J Surg Res. 1989;47:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 101. | Tiscornia O, Palasciano G, Sarles H. Effects of chronic ethanol administration on canine exocrine pancreatic secretion. Digestion. 1974;11:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Sarles H, Figarella C, Tiscornia O. Alcoholic pancreatitis. Mt Sinai J Med. 1975;42:540-551. [PubMed] |
| 103. | Tiscornia OM, Palasciano G, Sarles H. Atropine and exocrine pancreatic secretion in alcohol-fed dogs. Am J Gastroenterol. 1975;63:33-36. [PubMed] |
| 104. | Celener D, de la Porte P, Tiscornia O, Sarles H. Histochemical study of cholinergic activities in exocrine pancreas of dogs. Modifications related to chronic alcoholism. Biomedicine. 1977;27:161-165. [PubMed] |
| 105. | Sarles H, Tiscornia O, Palasciano G. Chronic alcoholism and canine exocrine pancreas secretion. A long term follow-up study. Gastroenterology. 1977;72:238-243. [PubMed] |
| 106. | Tiscornia OM. Pancreatitis crónica: Etanoly desequilibrio neuro endocrino. Medicina (. Bs. As.). 1997;37:187-190. |
| 107. | Perec CJ, Celener D, Tiscornia OM, Baratti C. Effects of chronic ethanol administration on the autonomic innervation of salivary glands, pancreas and heart. Am J Gastroenterol. 1979;72:46-59. [PubMed] |
| 108. | Baratti CM, Rubio MC, Perec CJ, Tiscornia OM. Effect of chronic alcohol feeding on adrenergic and cholinergic neurotransmission mechanism. Am J Gastroenterol. 1980;73:21-27. [PubMed] |
| 109. | Tiscornia OM, Celener D, Perec CJ, De Lehmann ES, Cresta M, Dreiling DA. Physiopathogenic basis of alcoholic pancreatitis: the effects of elevated cholinergic tone and increased "pancreon" ecbolic response to CCK-PZ. Mt Sinai J Med. 1983;50:369-387. [PubMed] |
| 110. | Rubio MC, Perec CJ, Medina JH, Tiscornia OM. Effects of chronic ethanol feeding on sympathetic innervated organs: temporal sequence of biochemical, functional, and trophic changes. Alcohol Clin Exp Res. 1984;8:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 111. | Perec CJ, Tiscornia OM, Baratti CM, Tumilasci OR, Dreiling DA. Trophic, biochemical and functional changes in submaxillary glands and pancreas induced by chronic alcohol feeding as indirect effect mediated by parasympathetic autonomic centers. Mt Sinai J Med. 1984;51:664-674. [PubMed] |
| 112. | Vaccaro MI, Tiscornia OM, Calvo EL, Cresta MA, Celener D. Effect of ethanol intake on pancreatic exocrine secretion in mice. Scand J Gastroenterol. 1992;27:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | Tiscornia OM, Perec CJ, Celener D, Cresta MA, Tumilasci OR, de Lehmann ES, Dreiling DA. The relationship of hyperactivity of the duodenal autonomic nervous brain and enhanced "pancreon" secretory response to CCK-PZ in chronic alcoholism. Mt Sinai J Med. 1984;51:650-663. [PubMed] |
| 114. | Tiscornia OM, Dreiling D, Vaccaro MI, Negri G, Celener D, Calvo E, Cresta MA, Perec C. Hipótesis fisiopatogénica de la pancreatitis alcohólica. Medicina (. Bs. As.). 1986;46:616-624. |
| 115. | Tiscornia OM, Dreiling DA. Physiopathogenic hypothesis of alcoholic pancreatitis: supranormal ecbolic stimulation of the "pancreon" units secondary to the loss of the negative component of pancreas innervation. Pancreas. 1987;2:604-612. [PubMed] [DOI] [Full Text] |
| 116. | Tiscornia OM, Celener D, Vaccaro MI, Cresta MA, Waisman H. [Acute pancreatitis: physiopathogenic hypothesis of fat necrosis]. Medicina (. B Aires). 1988;48:530-542. [PubMed] |
| 117. | Tiscornia-Wasserman PG, Tiscornia OM, Rybak BJ, Dreiling DA. Acute pancreatitis in a patient treated for alcoholic hepatitis. The hypothesis of supranormal ecbolic stimulation of the pancreon. Int J Pancreatol. 1989;4:345-352. [PubMed] |
| 118. | Tiscornia OM, Celener D, Cresta MA, Negri G, Vaccaro MI, Bustos Fernndez L. El alcoholismo crónico descentraliza autonómicamente al pncrease incrementa la reactividad de los centros neurales periféricos que modulan su secreción exocrina. Arch Arg Enf Ap Digest. 1991;5:143-172. |
| 119. | Brugge WR, Burke CA, Brand DL, Chey WY. Increased interdigestive pancreatic trypsin secretion in alcoholic pancreatic disease. Dig Dis Sci. 1985;30:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Yamasaki K, Okazaki K, Sakamoto Y, Yamamoto Y, Yamamoto Y, Okada T. Effects of ethanol on the motility of papillary sphincter and exocrine pancreas in the monkey. Am J Gastroenterol. 1993;88:2078-2083. [PubMed] |
| 121. | Guelrud M, Mendoza S, Rossiter G, Gelrud D, Rossiter A, Souney PF. Effect of local instillation of alcohol on sphincter of Oddi motor activity: combined ERCP and manometry study. Gastrointest Endosc. 1991;37:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 122. | Pitchumoni CS, Bordalo O. Evaluation of hypotheses on pathogenesis of alcoholic pancreatitis. Am J Gastroenterol. 1996;91:637-647. [PubMed] |
| 123. | Reber PU, Lewis MP, Kusske AM, Toyama MT, Ashley SW, Reber HA. Ethanol (EtOH) induces neutrophil activation and extravasation in the pancreas. Pancreas. 1995;11:445 (A). |
| 124. | Nevalainen TJ, Seppä A. Acute pancreatitis caused by closed duodenal loop in the rat. Scand J Gastroenterol. 1975;10:521-527. [PubMed] |
| 125. | Chetty U, Gilmour HM, Taylor TV. Experimental acute pancreatitis in the rat--a new model. Gut. 1980;21:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 126. | Rao SS, Watt IA, Donaldson LA, Crocket A, Joffe SN. A serial histologic study of the development and progression of acute pancreatitis in the rat. Am J Pathol. 1981;103:39-46. [PubMed] |
| 127. | Brackett KA, Crocket A, Joffe SN. Ultrastructure of early development of acute pancreatitis in the rat. Dig Dis Sci. 1983;28:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Dickson AP, Foulis AK, Imrie CW. Histology and bacteriology of closed duodenal loop models of experimental acute pancreatitis in the rat. Digestion. 1986;34:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Bockman DE. Early association of duodenal contents and blood with acini during experimental pancreatitis. Int J Pancreatol. 1988;3:333-342. [PubMed] |
| 130. | Tani S, Itoh H, Koide M, Okabayashi Y, Otsuki M. Involvement of endogenous cholecystokinin in the development of acute pancreatitis induced by closed duodenal loop. Pancreas. 1993;8:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 131. | Furukawa M, Kimura T, Yamaguchi H, Kinjoh M, Nawata H. Role of oxygen-derived free radicals in hemorrhagic pancreatitis induced by stress and cerulein in rats. Pancreas. 1994;9:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 132. | Orda R, Hadas N, Orda S, Wiznitzer T. Experimental acute pancreatitis. Inducement by taurocholate sodium-trypsin injection into a temporarily closed duodenal loop in the rat. Arch Surg. 1980;115:327-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 133. | De Rai P, Franciosi C, Confalonieri GM, Biffi R, Andreoni B, Uggeri F, Malesci A. Effects of somatostatin on acute pancreatitis induced in rats by injection of taurocholate and trypsin into a temporarily closed duodenal loop. Int J Pancreatol. 1988;3:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Tiscornia OM, García H, Hamamura S, Lehmann ES, de , González E, Vaccaro MI, Cerini C, Waisman H. Pancreatitis Aguda Biliar: rol pivote del sistema nervioso autónomoy de la disrupción del feedback entero-pancreático. Influencia del alcoholismo. Simil experimental con el método de Pfeffer. Valor preventivoy terapéutico de los anestésicos locales. Pren Méd Argent. 1998;85:494-503. |
| 135. | Isogai M, Yamaguchi A, Hori A, Nakano S. Hepatic histopathological changes in biliary pancreatitis. Am J Gastroenterol. 1995;90:449-454. [PubMed] |
| 136. | Varela López JA. “El Sondeo Gastro-Duodenal”. Edited by Centro de Gastroenterologia. Hospital Maciel, Montevideo, Uruguay. In: “Emergencias Médicasy Quirúrgicas”. Edited by Barè G, Bernabó J, Califano J and Waisman H. Published by EDIMED, Buenos Aries 1948; . |
| 137. | VARELA LOPEZ J, VARELA FUENTES B, MARTINEZ PRADO G. [5 Stages of normal duodenal tube and their modifications in diseases of the gallbladder and bile ducts]. Arch Mal Appar Dig Mal Nutr. 1950;39:797-810. [PubMed] |
| 138. | Suárez CV. Ampulla of Vater-a misnomer. Mt Sinai J Med. 1980;47:373-385. [PubMed] |
| 139. | Suarez CV. Structure of the major duodenal papilla. Mt Sinai J Med. 1982;49:31-37. [PubMed] |
| 140. | Singer MV, Solomon TE, Wood J, Grossman MI. Latency of pancreatic enzyme response to intraduodenal stimulants. Am J Physiol. 1980;238:G23-G29. [PubMed] |
| 141. | Zabielski R, Onaga T, Mineo H, Kato S, Pierzynowski SG. Intraduodenal cholecystokinin octapeptide (CCK-8) can stimulate pancreatic secretion in the calf. Int J Pancreatol. 1995;17:271-278. [PubMed] |
| 142. | Cunningham ME, Shaw-Stiffel TA, Bernstein LH, Tinghitella TJ, Claus RE, Brogan DA, McMillen MA. Cholecystokinin-stimulated monocytes produce inflammatory cytokines and eicosanoids. Am J Gastroenterol. 1995;90:621-626. [PubMed] |
| 143. | McCafferty DM, Sharkey KA, Wallace JL. Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol. 1994;266:G560-G567. [PubMed] |
| 144. | Miah A, Bank S, Stark B, Tiscornia OM; The effect of pre ERCP local anesthetic spray of the ampulla on the ease of cannulation and post ERCP hyperamylasemia and pancreatitis. Digestive Disease Week, Orlando, Florida, Mayo 16-19, 1999. Abstract M, 2973, pág: A-539. . |
| 145. | Tiscornia OM, Lehmann ES. de, Hamamura S, Otero G, Waisman H, Tiscornia Wasserman P. “Short Term”, “Closed Duodenal Loop” in the Rat: A Suitable model to elicit autonomic arc reflexes and mimick human bilary acute pancreatitis. Benefical effects of previous intraduodenal Lidocaine instillation. Am J Gastroenterol. 1999;94:2638 (A). |
| 146. | Soda K, Shimanuki K, Yoshida Y, Seo N, Yamanaka T, Sakurabayashi I, Miyata M. Serum lidocaine and MEGX concentrations after pharyngeal anesthesia for gastroscopy. Endoscopy. 1994;26:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 147. | Lewis MP, Kusske AM, Reber PG, Toyama MT, Ashley SW, Reber HA. Increased tissue myeloperoxidase activity in the feline pancreas after ischemia reperfusion. Pancreas. 1995;11:437 (A). |
| 148. | Brodmerkel GJ, Kaw M, Balu R, Ahn J, Mercer D, Ravi TJ, Agrawal R. Serum interleukin 6 (IL-6) levels in ERCP-induced pancreatitis. Pancreas. 1995;11:423 (A). |
| 149. | Norman J, Franz M, Messina J, Riker A, Fabri PJ, Rosemurgy AS, Gower WR. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 150. | Banks PA, Carr-Locke DL, Slivka A, Van Dam J, Lichtenstein DR, Hughes M. Urinary trypsinogen activation peptides (TAP) are not increased in mild ERCP-induced pancreatitis. Pancreas. 1996;12:294-297. [PubMed] |
| 151. | Lezcano H, Delgado JR. Farmacologia de los anestésicos locales. Rev Arg Anest. 1995;53:27-33. |
| 152. | Aho HJ, Nevalainen TJ, Lindberg RL, Aho AJ. Experimental pancreatitis in the rat. The role of phospholipase A in sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 153. | Hughes CB, el-Din AB, Kotb M, Gaber LW, Gaber AO. Calcium channel blockade inhibits release of TNF alpha and improves survival in a rat model of acute pancreatitis. Pancreas. 1996;13:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 154. | Tiscornia OM, Hamamura S, Lehmann ES, de , González E, Vaccaro MI, Otero G, Cerini C, Waisman H. La inervación automómica gastro entero bilio pancre tica. El concepto de “pista” plexual entérica. Pren Méd Argent. 1999;86:129-139. |
| 155. | Tiscornia OM, García H, Affani JM, Otero G, Tiscornia Wasserman P. Blood changes in acute pancreatitis induced by balloom disteation of the PeriVaterian Duodenum in the Opossum and the effects of previous truncal vagotomy and bilateral splanchnicectomy. Am J Gastroenterol. 1999;94:2638 (A). |