Published online Feb 15, 2000. doi: 10.3748/wjg.v6.i1.89
Revised: August 22, 1999
Accepted: September 11, 1999
Published online: February 15, 2000
AIM: To observe the tumor inhibitory effects by transfecting I L-6 cDNA into colon cancer cell line HT-29 with retroviral vector pZIP cDNA.
METHODS: Human IL-6 gene was reconstructed in retrovirus vector and transfected into incasing cells PA317 by lipofectamine mediated method, the clones of the cells transferred with hIL-6 were selected by G418, and targeted HT-29 cells were infected with the virus granules secreted from PA317 and also selected by G418. Test gene transcription and expression level by hybridization , ELISA and MTT assay, etc. Analyze tumor inhibitory effects according to the cell growth curve, plating forming rate and tumorigenicity in nude mice.
RESULT: Successfully constructed and transfected recombinant exp ressing vectors pZIPIL-6 cDNA and got positive transfected cell lines. The colon cancer cell line (HT-29 IL-6) transfected with the hIL-6 gene by retroviral vector was established. The log proliferation period and the doubling time of t his cell line was between 4 to 7 days and 2.5 days according to the direct cell count, the cell proliferation was obviously inhibited with MTT assay, the plating inhibitory rate was 50% by plating efficiency test. When HT-29 IL-6 cells w ere inoculated into the nude mice subcutaneously, carcinogenic activity of the solid tumor was found superior to the control group and the size of tumor was not significantly enlarged. Injection of combination virus fluid containing IL-6 gene into transplantation tumors could inhibit the growth and development of the tumor.
CONCLUSION: IL-6 could inhibit the growth and proliferation of colon cancer cells by retroviral vector-mediated transduction.
- Citation: Xiao B, Jing B, Zhang YL, Zhou DY, Zhang WD. Tumor growth inhibition effect of hIL-6 on colon cancer cells transfected with the target gene by retroviral vector. World J Gastroenterol 2000; 6(1): 89-92
- URL: https://www.wjgnet.com/1007-9327/full/v6/i1/89.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i1.89
The incidence of colorectal carcinoma ranks top ten among the digestive system diseases. The clinical treatment so far is not satisfactory, which has been exis ted of the top ten malignancy threatening human life. The authors intended to use modern molecular biology technology to explore the new strategies in treatment of colorectal carcinoma. IL-6 is a multifunctional cytokine which has been con firmed to have anti-tumor effect[1]. Here, we reported a colorectal car cinoma cell line transfected with IL-6 gene, which can secret a large amount of IL-6 and study its tumor-inhibitory effect both in vitro and in vivo.
The colorectal carcinoma cell line ( HT-29 cell strain) was preserved in our lab oratory, and PA317 package cell was generously provided by Professor Zhang Hong -Quan from the Academy of Military Medical Sciences of China.
Restrict enzyme, EcoRI, BamHI, Klenow enzyme and T4 DNA ligase were purchased from Sino-American Biotechnology Company (SABC).
pUCIL-6cDNA, pZIPcDNA was introduced Dr. Jiang Bo from the Academy of Military Medical Sciences of China.
Twenty BALB/c-nu/nu female nude mice, 3-4 weeks old, were purchased from Shanghai Tumor Institute.
By utilizing restrict enzyme digestion and DNA recombinant technology, we constructed a recombinant retroviral plasmid which was named pZIPIL-6cDNA. Then the plasmid was transfected into PA317 package cell by using liposome[2]. After screening the positive (anti-drug) clones with G418, we collected supernants of positive clones containing recombinant virus and transfected the adenocarcinoma HT-29 cell line with the supernatant and screening of drug-resistance clones by G418.
According to the direct count assay[3], the log proliferation and the doubling time of HT-29 cells transfected with or without pZIPIL-6cDNA were calcu lated. The proliferation rate of the two groups was cultured in 96-well plate and observed by MTT assay at 48 h, 72 h, 96 h and 120 h, respectively[4].
Prepared double layer agar gel in 6-well plate (soft agar layer) containing 0.3% agar, 20% BSA; the lower layer containing 0.5% agar, 10% BSA, which was refe rred as hard agar layer. Then different amount of 2 × 103 HT-29/IL-6 cells or HT-29 cells was added per well to continue for 3-4 weeks, the colony formation efficiency was calculated, including the number of the average colonization unit/the number of cell added per well.
Twenty mice were randomly divided into four groups and each five mice. The first two groups received inoculation of HT-29 cells. After tumor formation, one group was injected pZIPIL-6cDNA recombinant viral suppressant intratumorally, which was called IL-6 gene therapy group. The other served as control group. The other two groups were inoculated with pZIPcDNA or pZIPIL-6cDNA transfected HT-29 cells, respectively. 5 × 10 6 cells were injected at left front leg subcutaneously. Then observed after forty days and 6 items were recorded including the time of tumor formation (i.e. the time when tumor diameter was about 3 mm), the number of tumor-bearing mice, tumor growth rate, lesion size, and health condition of nude mice as well as pathologic characteristic of the tumor.
After recombinant vector (pZIPIL-6) was transfected into PA317 package cells, the positive clones were screened for 4-5 weeks and drug-resistant clones gradu ally formed, at the same time untransfected cells died completely after 1-2 wee ks and no colonization was found. The recombinant viral suppressant titer was 5.1 × 108 cfu/L by using 3T3 cells as indicating system[2].
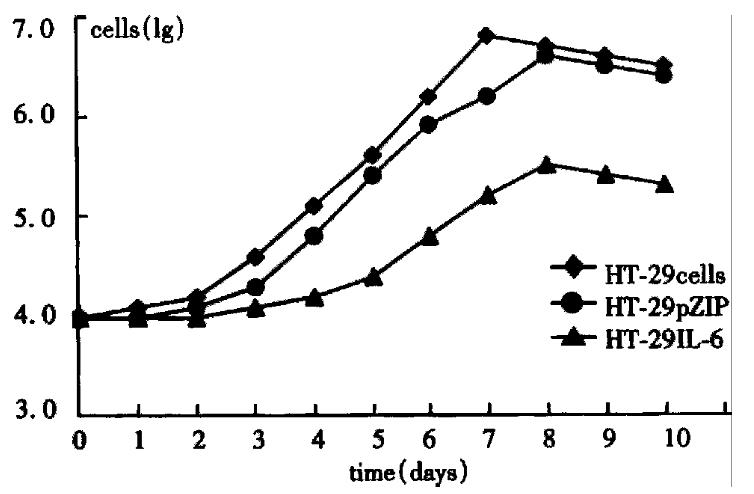
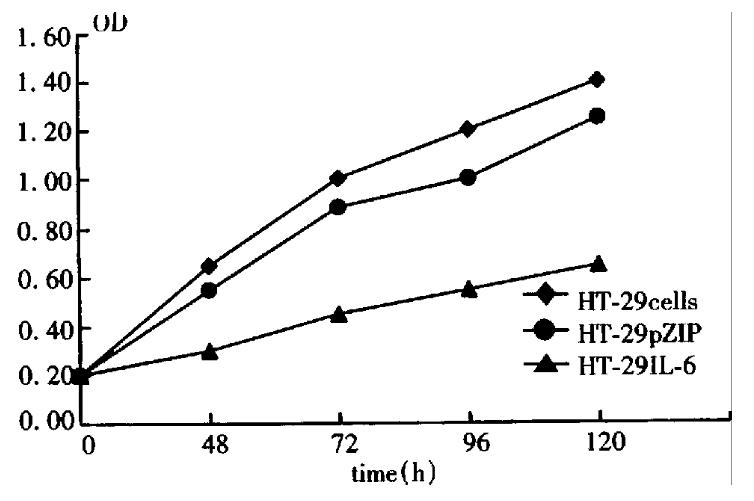
By direct counting assay, the difference in growth between HT-29 cells and HT-29 IL-6 cells is shown in Figure 1, the doubling time of HT-29 and HT-29 pZIP was 1.5 and 1.6 days, respectively, and the log proliferation phase was 2-7 days and 3-8 days, respectively. But the doubling time of HT-29 IL-6 cells was 2.5 days, log proliferation phase was 4-7 days, with a maximal cell number of 2.5 × 10 5, which was significantly lower than HT-29 cells. The results of MTT assay are shown in Figure 2, in which the cell proliferating curve of HT-29 IL-6 cells increased more slowly being more stabilized than other groups. These demonstrated that IL-6 inhibited the growth and proliferation of colon cancer cells (HT-29) significantly, and retroviral vector influenced neither the proliferation of HT-29 cells nor the biologic effect of IL-6.
The HT-29 cell and HT-29 IL-6 cell clones were formed gradually, between 12-14 days after inoculation on the soft agar, and a number of them only appeared 3-4 weeks after. A single cluster with more than 50 cells was regarded as a clone unit, and the efficiencies of colonization were calculated (Table 1). The efficiencies for HT-29 IL-6 cells and HT-29 cells were 2.21% and 4.29%, respectively, this demonstrated the clone inhibitory rate was 50% for transfected cells. Therefore, it was shown that transfected IL-6 gene could inhibit the ca pacity of HT-29 colonization. It might be of significance in the prevention of HT-29 tumor cell from proliferating and invasion.
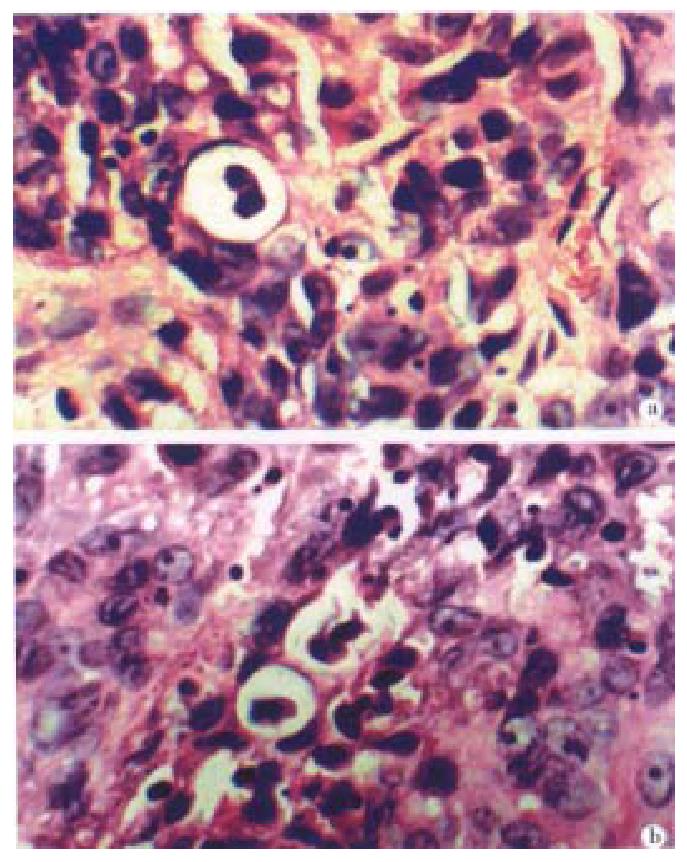
Local edema disappeared inoculation after 2-3 days. After 8-14 days there appeared tiny tumors which enlarged continuously (Table 2). The average tumor formation time of gene-transfected group was delayed 5.5 days as compared with the control group, and one mouse did not show any sign of tumor bearing. In IL-6 treated mice, the tumor grew slower, the health condition was much better, and no cachexia was seen, in particular in the intratumorously-injected mice. Pathology sections showed there was more distinct apoptosis (Figure 3).
Recently, pioneered studies showed that IL-6 could promote or inhibit the growth of tumor cell depending upon the cell type[5]. For example, the IL-6 cDNA encoding sequences were inserted into the downstream of SV40 promoter in pH EBOSV plasmid, then the plasmid was transfected into ROHA-9MCEBV3-B cell. The observation showed that exogenous IL-6 gene expression could switch the cell to a tumor phenotype. 10-15 days after the injection of transfected ROHA-9MCEBV3 -B cell, tumor formations were observed, but the control group was not, which suggested that IL-6 secreted by the transfected cell promote cell proliferation in carcinogenesis. On the other hand, administration of IL-6 alone could in hibit the metastasis of tumor induced by xylene anthracene[6]. Currently, the mechanism remained unknown, however, it may be associated with different IL-6R expression on tumor cell surface. Only when the latter reaches a certain level and binds to IL-6 with high affinity could it transmit I L-6 signals and showed significant inhibitory activity. IL-6 is a cytokine with multifunctions as stated above. For instance, when the IL-6 gene was transfected into D122 Lewis lung cancer cell line which has low immunity and high metastatic potential, growth of transfected cell was inhibited in vitro, and the density of MHC antigen expressed on cell surface was 2.5-4.5 times greater than that in the wild type of D122. Immunizing the mice with inactivated transfected cells, the mice showed an enhanced activity of CTL and macrophage, the immunization could prevent mice from challenge of wild type D122 cells. These phenomena d emonstrated that IL-6 not only could inhibit tumor growth directly but also pro mote anti-tumor activity indirectly. Through activating host cellular immunity[7]. Ullmann et al[8] found that IL-6 could enhance CEA and HLA antigen expression on colon cancer cell surface and these antigens expression level correlated with IL-6 treatment dose and duration positively. Other reports showed that CEA expression level of IL-6 gene transfected into colon cancer cell line (HT-29 cells IL-6) was much higher than that of untransfected or transfected with unrelated gene cells[9]. Apart from these studies, IL-6 could enhance the induction of LAK cell or NK cell, promote the maturity and differentiation process, and increase the tumor killing activity of LAK and NK cells in a dose-dependent manner. Overall, introducing IL-6 gene into cells may have wide biological effects on the regulation of immunological response by autocrine or paracrine pathways, thus inhibit or kill tumor cell directly or indirectly[10,11]. Regulatory disturbance induced by apoptosis is closely associated with carcinogenesis, making tumor apoptosis a new target for cancer therapy[12]. Gene regulation and signal tranduction are the key process of apoptosis. Tumor cells could escape surveillance of immune system, not only because of the downregulation of antigen expressed on cell surface and host immune response, but also the inhibition of apoptosis of abnormal cell. We reported the first time that many more apoptotic cells in the group injected with HT-29 IL-6 cells and IL-6 gene therapy group were induced than the control group. So far, we have not see n any report on apoptosis induced by IL-6, even some researchers reported IL-6 could inhibit the programmed cell death in MI leukemia cell strain[13]. IL-6 is a potent immunoregulatory factor, and participates in regulation of cell apoptosis. Thus, we conclude the apoptosis induction of tumor cell is the main mechanism of IL-6 for growth inhibition of tumor cells both in vitro and in vivo. Our laboratory studies provided the potential use of IL-6 gene therapy in colo-rectal cancer.
Edited by Wu XN
Proofread by Miao QH
| 1. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: labora-tory manual (2nd ed). New York: Cold Spring Harbor Laboratory Press. 1992;34-80. [Cited in This Article: ] |
| 2. | Jiang B, Zhang YL, Zhou DY. The laboratory methods used in molecular biology. 1st ed. Beijing: The People's Military Surgeon Press. 1996;157-162. [Cited in This Article: ] |
| 3. | Jiang B; EZ. Tissue culture techniques. 2nd ed. Beijing: The Poeple's Public Health Press. 1993;70-120. [Cited in This Article: ] |
| 4. | Siitonen SM, Kallioniemi OP, Isola JJ. Proliferating cell nuclear antigen immunohistochemistry using monoclonal antibody 19A2 and a new antigen retrieval technique has prognostic impact in archival paraffin-embedded node-negative breast cancer. Am J Pathol. 1993;142:1081-1089. [PubMed] [Cited in This Article: ] |
| 5. | Lu XY, Wang ZC, Yang GZ. The relationships between IL-1, IL-6 and tumor. Guowai Yixue Shengli Bingli Kexue Yu Linchuang Fence. 1995;15:159-163. [Cited in This Article: ] |
| 6. | Mulé JJ, McIntosh JK, Jablons DM, Rosenberg SA. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med. 1990;171:629-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 177] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Porgador A, Tzehoval E, Katz A, Vadai E, Revel M, Feldman M, Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992;52:3679-3686. [PubMed] [Cited in This Article: ] |
| 8. | Ullmann CD, Schlom J, Greiner JW. Interleukin-6 increases carcinoembryonic antigen and histocompatibility leukocyte antigen expression on the surface of human colorectal carcinoma cells. J Immunother (1991). 1992;12:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Tsang KY, Kashmiri SV, Qi CF, Nieroda C, Calvo B, De Filippi R, Greiner JW, Primus FJ, Schlom J. Transfer of the IL-6 gene into a human colorectal carcinoma cell line and consequent enhancement of tumor antigen expression. Immunol Lett. 1993;36:179-185. [PubMed] [Cited in This Article: ] |
| 10. | Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1320] [Cited by in F6Publishing: 1391] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 11. | Jeana C. Treatment with IL-6 in cancer and inflammation. Guowai Yixue Mianyixue Fence. 1992;12:216-217. [Cited in This Article: ] |
| 12. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 16] [Reference Citation Analysis (0)] |
| 13. | Xiang LB, Lin YL, Xiang JM. Molecular biology in programmed cell death. Manyixue Zazhi. 1995;11:71-76. [Cited in This Article: ] |