Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.531
Revised: August 20, 1999
Accepted: September 15, 1999
Published online: December 15, 1999
- Citation: Wan R, Gao MQ, Gao LY, Chen BF, Cai QK. In situ detection of Epstein-Barr virus in gastric carcinoma tissue in China highrisk area. World J Gastroenterol 1999; 5(6): 531-532
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.531
Epstein-Barr virus ( EBV ), a gammaherpesvirus, has been strongly associated with African Burkitt’s lymphoma and nasopharyngeal carcinoma. Recently it has been identified in lymphoepithelioma-like carcinoma of thymus, tonsil, lung and in some gastric carcinoma[1-4]. The development of very sensitive methods for detection of EBV infection in archival pathologic tumor sections has allowed us to study the association of EBV with gastric adenocarcinomas by using In situ hybridization with EBER-1 oligoprobes and immunohistochemistry with anti-LMP1 antibodies.
Cases were selected from a series of primary gastric carcinomas collected at the Department of Pathology, Fujian Medical University, Fuzhou, Fujian. The specimens included 58 primary gastric carcinomas, 5 chronic peptic ulcer and 10 additional specimens of normal gastric mucosa obtained from postmortem patients without gastrointestinal disease. Formalin-fixed, paraffin-embedded tissues were prepared for light microscopic examination.
In situ hybridization The EBV sequence, EBER-1, was detected with a complementary digoxigeninlated 30-base oligomer using a procedure proviously described[5]. A blue-brown or brown color within the nucleus over background levels was considered positive. In each case, hybridization was applied to section that contained both neopla stic and adjacent non-neoplastic mucosa.
A known EBV-positive nasopharyngeal carcinoma served as positive control and In situ hybridization without EBER probe was taken as negative control.
Paraffin-embedded sections were stained with monoclonal antibodies ( CS1-4, DAKO ) to evaluate the expression of LMP-1. Immunostaining was performed with the avidin-biotin complex (ABC) method as previously described. For these antibodies, the method of antigen retrieval was used by microwave oven pretreatment in place of proteolytic digestion before immunostaining. LMP-1 positive nasopharynge al carcinomas were used as positive controls.
The age range of patients was 37-74 years, with a median age of 54.5 years. Fifty patients were males and 8 were females, the ratio of males to females being 6.3:1. Among the 58 gastric adenocarcinomas, 22 were poorly-diffe rentiated, 18 tubular, 10 mucinous, 7 signet-ring cell carcinoma, 1 papillary a denocarcinoma.
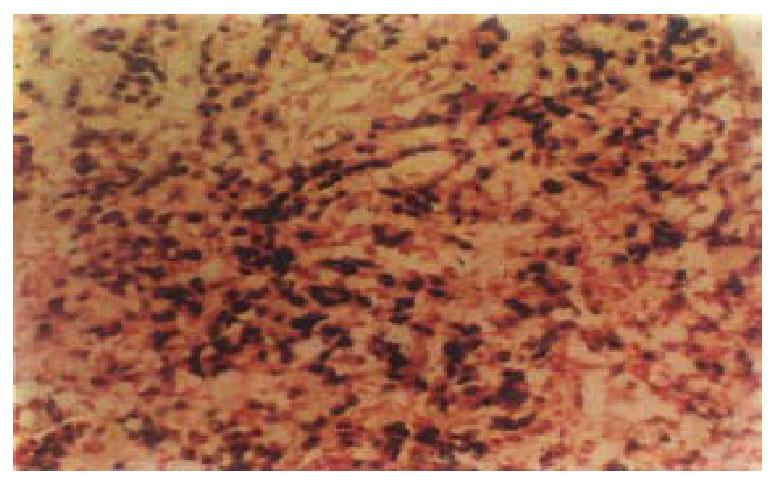
EBER-1 expressionIn situ hybridization, signals were stro ng and limited to the nucleus of carcinoma cell (Figure 1). A blue-brown or brown color was considered a positive signal. Six cases ( 10.3% ) showed EBER-1 exp ression, including 5-poorly-differentiated and 1 papillary adenocarcinoma. In positive cases, virtually all malignant cells were strongly labeled with EBER pro bes, while the infiltrating lymphocytes, blood vessels and smooth muscle were EB ER-1 negative. No EBER-1 signals were observed in adjacent non-neoplastic epithelial cells, dysplastic epithelial cells and normal gastric mucosa.
LMP-1 expression No expression of LMP-1 was seen in both EBER-1 positive and negative cases.
EBER-1 is EBV encoded small RNA, of which high levels expression can be up to 106-107 copies per cell exceeding DNA in EBV-infected cell. EBER oligoprobe may combine with EBER-1 enabling for detecting EBV on paraffin-embedded tissues using non-isotopic labeling and In situ hybridization technique which are now considered the most sensitive methods.
EBV is an oncogenic virus which has neoplastic transforming properties. The incidence of EBV infection is high in Chinese population especially in east and south China. In the current study, we had demonstrated the presence of viral RNA in 10.3% of our cases of gastric carcinomas by this method. The frequency of EBV gene expression in gastric malignant epithelium in Fuzhou was higher than that (1.6% to 6.1%) reported in Changsha and Shenyang[6]. Our study suggested that the frequency of EBV infection was different in different regions of China.
The presence of EBV in neoplasms seemed to be related to the histological subtype of neoplasms. Raab-Traub et al[7], found EBV in undifferentiated and poorly differentiated nasopharyngeal carcinomas, which generally contained a relatively large number of EBV genome equivalents. In Hodgkin’s disease, presence of EBV was often in mixed cellularity ( HD-MC, 91% ) and nodular sclerosis (HD-NS, 43%) subtypes[8]. In our study, most of the EBV-postive cases were poorly differentiated gastric carcinomas. The relation between EBV infection and poorly differentiated carcinomas is unknown. Many believed that EBV virus might multiply easier in the poorly differentiated carcinoma, EBV genome amplification favored growth of malignant cells, promote infiltration and metastasis[7]. We failed to detect LMP-1 expression in this tumor due to the methylation of coding and regulatory regions of this protein[9].
Shibata et al[10] and Gulley et al[11], found that the EBER-1 was present in dysplastic epithelium of gastric mucosa before malignancy transformation. But our result showed no EBER-1 expression in the adjacent non-neoplastic epithelium, dysplastic cells and normal gastric mucosa. Finally low frequency of Eps tein-Barr virus in gastric carcinoma suggests that EBV does not play any import ant role in the pathogenesis of gastric carcinoma.
Edited by Xie-Ning Wu
Prooofread by Qi-Hong Miao
| 1. | Dimery IW, Lee JS, Blick M, Pearson G, Spitzer G, Hong WK. Association of the Epstein-Barr virus with lymphoepithelioma of the thymus. Cancer. 1988;61:2475-2480. [PubMed] |
| 2. | Brichácek B, Hirsch I, Síbl O, Vilikusová E, Vonka V. Presence of Epstein-Barr virus DNA in carcinomas of the palatine tonsil. J Natl Cancer Inst. 1984;72:809-815. [PubMed] |
| 3. | Kasai K, Sato Y, Kameya T, Inoue H, Yoshimura H, Kon S, Kikuchi K. Incidence of latent infection of Epstein-Barr virus in lung cancers--an analysis of EBER1 expression in lung cancers by in situ hybridization. J Pathol. 1994;174:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Shibata D, Tokunaga M, Uemura Y, Sato E, Tanaka S, Weiss LM. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469-474. [PubMed] |
| 5. | Hamilton-Dutoit SJ, Raphael M, Audouin J, Diebold J, Lisse I, Pedersen C, Oksenhendler E, Marelle L, Pallesen G. In situ demonstration of Epstein-Barr virus small RNAs (EBER 1) in acquired immunodeficiency syndrome-related lymphomas: correlation with tumor morphology and primary site. Blood. 1993;82:619-624. [PubMed] |
| 6. | Wang M, Tokunaga M, Jia XS. [Observations on the relation of gastric carcinoma to Epstein-Barr virus]. Zhonghua Binglixue Zazhi. 1994;23:285-287. [PubMed] |
| 7. | Raab-Traub N, Flynn K, Pearson G, Huang A, Levine P, Lanier A, Pagano J. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. Int J Cancer. 1987;39:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Zhou XG, Hamilton-Dutoit SJ, Yan QH, Pallesen G. High frequency of Epstein-Barr virus in Chinese peripheral T-cell lymphoma. Histopathology. 1994;24:115-122. [PubMed] |
| 9. | Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci USA. 1994;91:9131-9135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 337] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769-774. [PubMed] |
| 11. | Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996;27:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 4.2] [Reference Citation Analysis (0)] |