Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.133
Revised: December 20, 1997
Accepted: February 20, 1998
Published online: April 15, 1998
AIM: To evaluate the curative effect of stage II surgical resection of hepatocellular carcinoma after TAE.
METHODS: Thirty-eight patients with unresectable hepatocellular carcinoma were treated by transcatheter arterial embolization (TAE). When the sizes of tumors were markedly reduced after TAE, stage II surgical resections were performed.
RESULTS: Before TAE, the diameters of tumors were 12.84 cm ± 4.87 cm (x ± s), but reduced to 5.12 cm ± 1.82 cm (x ± s) after TAE (P < 0.001). Pathologic examination of the resected specimens revealed obvious necrosis in most cases. After surgery, 26 patients were alive, with the longest survival of 96 months, twelve died and 10 had tumor recurrence.
CONCLUSION: Patients in moderate and advanced stages of hepatocellular carcinoma after TAE should be treated surgically, but the indication must be controlled strictly.
- Citation: Wang JH, Lin G, Yan ZP, Wang XL, Cheng JM, Li MQ. Stage II surgical resection of hepatocellular carcinoma after TAE: a report of 38 cases. World J Gastroenterol 1998; 4(2): 133-136
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.133
Transcatheter arterial embolization (TAE) has been recognized as a new effective method for the treatment of moderate and advanced hepatocellular carcinoma[1-10]. Some patients are able to gain a chance of stage II surgical resection after TAE. But, what are the indications of stage II surgical resection? How about the curative effect? What are the influential factors? Up to date, there has been no report about these questions. A total of 1288 patients with advanced hepatocellular carcinoma were treated by TAE in our hospital from February 1987 to December 1992, 248 of them were followed up and 38 gained stage II surgical resection. The results are reported below in an attempt to explore the above mentioned questions.
This group consisted of 38 patients with hepatocellular carcinoma diagnosed pathologically. They were treated by TAE via catheterization of hepatic artery on account of no indication for surgery. TAE procedures were carried out alike as previously reported[1,6,9,10]. The duration from initial TAE treatment to stage II surgical resection in this group was 4 weeks to 16 months, averaging 6.38 months. TAEs were done 1 to 9 times for each patient with an average of 3.6 times and at intervals of 50-60 d. According to hepatic arteriographic findings, 28 of 38 cases were classified as huge mass type or huge mass plus nodular type (the diameter of tumor ≥ 10 cm), 10 as nodular type (the diameter of tumor 5 cm-9 cm) and none as diffuse type.
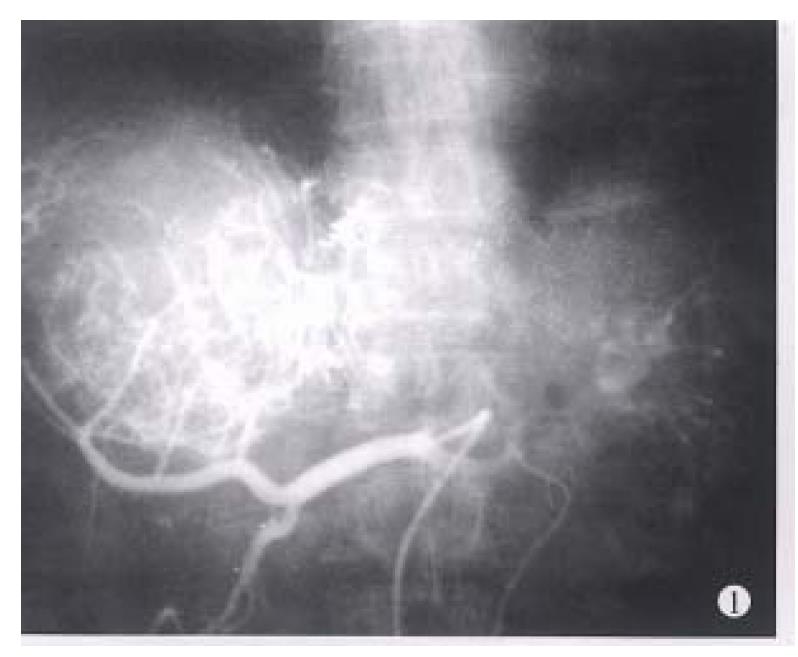
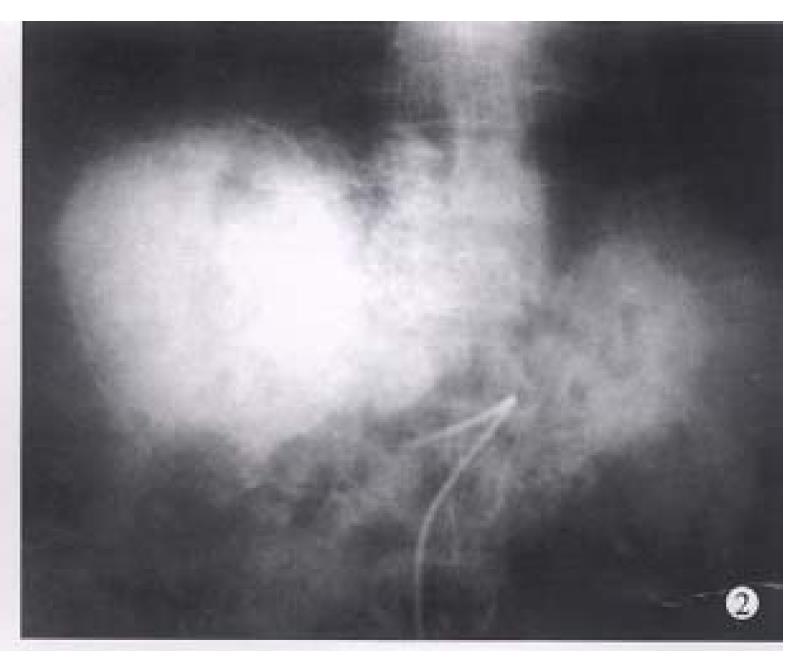
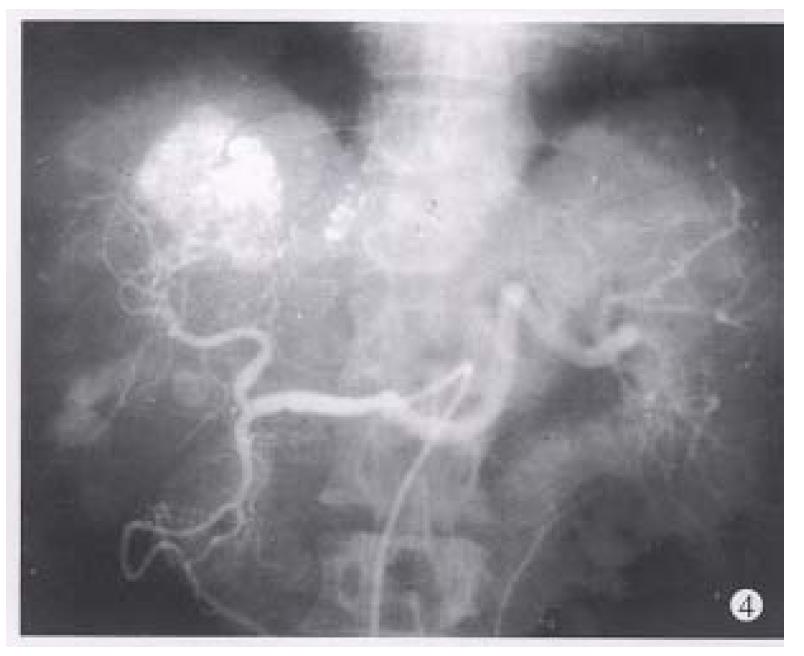
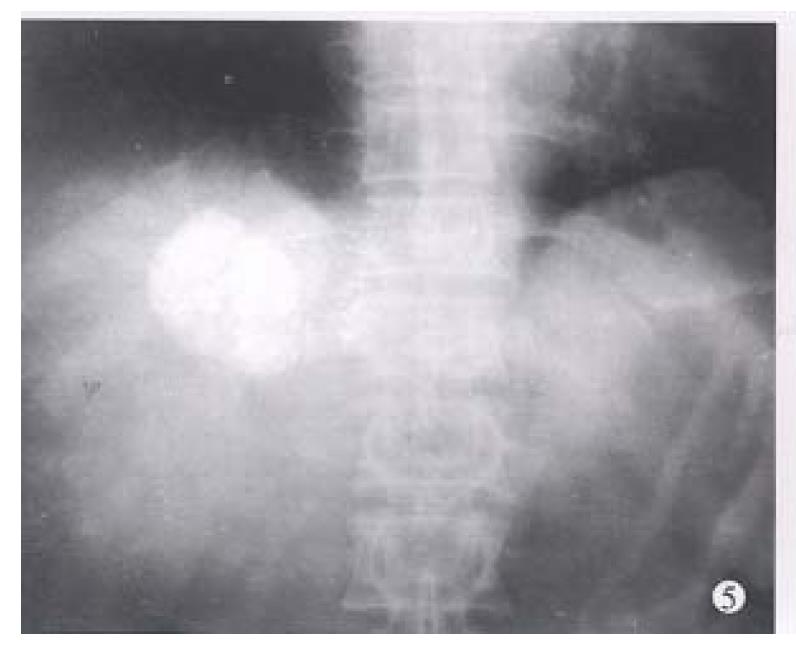
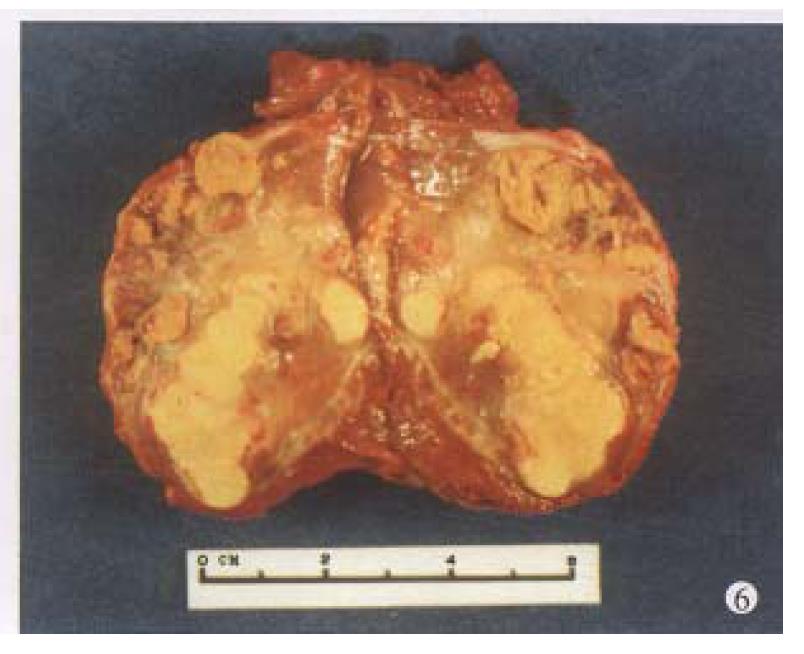
Before TAE, the diameters of tumors were 12.84 cm ± 4.87 cm (x-± s) and after TAE decreased to 5.12 cm ± 1.82 cm (x-± s), (P < 0.001) (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6). Tumors were removed in 36 cases, but not in the remaining 2 because of spreading to diaphragm and lesser epiploon, absolute ethanol was injected directly into the tumors with ligation of feeding hepatic artery. Liver tumor capsules were intact in 19 of 36 resected specimens observed with naked eyes, the capsules were not intact in 14, and none in 3. Under light microscope, there was complete necrosis of tumor cells in 12 cases, obvious necrosis in most parts of the tumor with a few living tumor cells in 20 cases, and partial necrosis with parts of tumor cells actively growing in 4 cases. There were inflammatory reaction and different degrees of fibrotic proliferation around the necrotic tissues. During operation, mild liver cirrhosis was seen in 11 cases while moderate and serious ones in 21 and 4 cases respectively, and none in 2.
So far, 26 of 38 patients have been alive, 22 of 26 being healthy. The average survival was 24.6 months, and the longest survival period has reached 96 months. Tumor recurred in 4 cases, 2 had bone metastasis one month after surgery, one was found to have multiple intrahepatic tumor nodules and one had elevated AFP values with right liver recurrent tumor of 4.5 cm × 5.2 cm in diameter, occurring 3 months and 19 months after surgery, respectively. Twelve patients died, 2 died separately from coronary arterial embolization and massive upper digestive tract bleeding one week after surgery, 8 died from recurrence of liver tumor, and the other 2 died of serious liver cirrhosis and liver function failure 4 and 16 months after surgery. The time between surgery and death was 4-28 months, with an average of 11.5 months (excluding 2 deaths one week after surgery).
Tumor recurred after surgery in 10 patients, 8 of the 10 recurrences were within liver, whereas 2 had bone metastasis. So far, 8 patients have died. Postoperative tumor recurrence time was 1 to 21 months with an average of 7.2 months. Cancerous emboli were found within the main trunks of portal vein or its large branches in the 10 patients, and 1 of them had also cancerous emboli in the right hepatic vein.
In our 38 patients, the sizes of liver tumors were markedly reduced after TAE. Pathologic examination of the resected specimens revealed obvious necrosis in most parts of tumor masses in nearly all the cases and complete necrosis in a few ones[6,9,10]. All these indicated the good therapeutic effects of TAE for hepatocellular tumor. The reason for the better results obtained in this group by TAE was the thorough embolization of tumor vessels and feeding arteries, as shown by the accumulation of large amount of lipiodol in all or most parts of tumor mass on CT scanning. Besides, most of the tumors in this group were of huge mass type or single nodule type. Furthermore, the lipiodol emulsion containing anticancer drugs obstructed the blood supplying to the tumors, resulting in ischemic necrosis[2,5,8] on one hand, and on the other hand, the lipiodol emulsion continued to release anticancer drugs for killing tumor cells which were already in an ischemic and more vulnerable state[6,9,10]. Therefore, the thoroughness of embolization for liver tumors directly influenced the effect. As far as the patients’ liver function would be tolerated, the embolization procedure should be undertaken to fully fill the tumor mass with lipiodol emulsion during each TAE, especially for the first time[9,10].
The surgical specimens from the 38 cases revealed that most parts of the liver tumors were necrotic, but a few living cancerous cells still remained, which might cause tumor recurrence or metastasis later[6,9,10]. On the other hand, after several times of TAE procedure, the main tumor feeding arteries had been obstructed and more collateral vessels thus formed, causing difficulties in super-selective catheterization via the collateral vessels and continuous TAE. The majority of patients were only treated by arterial infusion chemotherapy, which was usually ineffective in controlling tumor growth. Therefore, the liver tumor which had been markedly reduced in size after TAE should be removed further by stage II surgery. Thus, the prognosis would be much improved.
In this group, ten patients had liver tumor recurrence on an average of 7.2 months after stage II surgery (10/38, or 26.3%). There were three types of recurrences: ① 10 patients showed all cancerous emboli in the main trunks of portal veins and their bigger branches, with 1 case also having cancerous emboli in the right hepatic vein; ② in some cases, severaltumor nodules were still present besides the main tumor mass; and ③ in a few cases, merely palliative surgical resections were performed because the tumor sizes found during operation were still too large. Therefore, the indication for stage II surgical resection after TAE should be controlled strictly. The contraindications are as follows: hepatic arteriogram and CT scanning reveal main tumor mass with several satellite tumor nodes which can not be removed completely by surgery; the diameter of tumor is over 5 cm, which is only resected palliatively; cancerous emboli within the main trunk of portal vein or its large branches or large hepatic venous branches; distant metastasis; and serious liver cirrhosis.
| 1. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [PubMed] |
| 2. | Nakakuma K, Tashiro S, Hiraoka T, Uemura K, Konno T, Miyauchi Y, Yokoyama I. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983;52:2193-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Ohishi H, Uchida H, Yoshimura H, Ohue S, Ueda J, Katsuragi M, Matsuo N, Hosogi Y. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology. 1985;154:25-29. [PubMed] |
| 4. | Nakakuma K, Tashiro S, Hiraoka T, Ogata K, Ootsuka K. Hepatocellular carcinoma and metastatic cancer detected by iodized oil. Radiology. 1985;154:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Miller DL, O'Leary TJ, Girton M. Distribution of iodized oil within the liver after hepatic arterial injection. Radiology. 1987;162:849-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Kanematsu T, Furuta T, Takenaka K, Matsumata T, Yoshida Y, Nishizaki T, Hasuo K, Sugimachi K. A 5-year experience of lipiodolization: selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology. 1989;10:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, Maeda M, Yoshioka T. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:140-145. [PubMed] |
| 8. | Yamada R, Kishi K, Sonomura T, Tsuda M, Nomura S, Satoh M. Transcatheter arterial embolization in unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Lin G, Wang JH, Gu ZM, Yan ZP, Wang XL. Hepatic arterial infusion chemo-therapy and embolization in the treatment of moderate and advanced stages of hepatic carcinoma: therapeutic effects and some influential factors. Chin J Radiol. 1992;26:311-315. |
| 10. | Wang JH. [Hepatic arterial infusion chemotherapy plus embolization for unresectable liver cancer--a report on 40 patients]. Zhonghua Zhongliu Zazhi. 1992;14:276-279. [PubMed] |