Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.77
Revised: August 21, 1997
Accepted: October 2, 1997
Published online: February 15, 1998
AIM: To investigate the age-related alterations of cytoskeleton system in liver Kupffer cell and their relation to the changed phagocytic function.
METHODS: The phagocytic function of Kupffer cells from rats of various ages (6 mo, 12 mo,18 mo and 24 mo) were quantitatively evaluated by phagocytosis of polystyrene beads. The actin distribution and measurement of Kupffer cell were determined by a phalloidin-TRITC method; and the myosin and vimentin distribution and measurement with indirect immunochemical staining.
RESULTS: Aging resulted in significant alterations of actin, myosin and vimentin distributions and reductions in Kupffer cell; the 3 cytoskeleton components of 24-mo-old Kupffer cell were significantly decreased to 68.0%, 84.9% and 75.5%, respectively of these of 6-mo-old Kupffer cell(P < 0.01,0.01 and 0.01). And these decreases had significant positive relations with the damaged phagocytosis of the aged Kupffer cell. γ values were 0.96(P < 0.05), 0.99(P < 0.01) and 0.95 (P < 0.05) respectively.
CONCLUSION: The cytoskeleton system of the aged Kupffer cell presents an evident state of senescence, which may be an important mechanism of decreased phagocytosis of the aged Kupffer cell.
- Citation: Sun WB, Han BL, Peng ZM, Li K, Ji Q, Chen J, Wang HZ, Ma RL. Effect of aging on cytoskeleton system of Kupffer cell and its phagocytic capacity. World J Gastroenterol 1998; 4(1): 77-79
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/77.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.77
Kupffer cells account for about 30% of nonparenchymal liver cells and constitute the largest pool of resident macrophages in the body. They play an essential role in the elimination of foreign substance derived from the systemic circulation mainly through phagocytosis. The age-related alterations in Kupffer cell function are considered to be related to the susceptibility to sepsis after trauma or infection or to tumor of the old people[1]. In this study, the distribution and contents of actin, myosin and vimentin in Kupffer cells of various ages, and a quantitative evaluation of phagocytosis of polystyrene beads by primary cultured Kupffer cells of various ages will be described.
Twenty-four Wistar rats of different ages obtained from the Chinese Herb Research Institute of Sichuan Province were rendered into 4 groups (6, 12,18 and 24 months of age). Each group had 6 rats.
Liver nonparenchymal cells were isolated by a collagenase-perfusion method as reported previously[2]. Briefly, after anesthesia (30 mg of barbital/kg body wt., intraperitoneally), the liver was perfused in situ with Ca++ -free Hanks balanced salt solution at 37 °C for 3 min. Then 0.05% collagenase (Sigma, Type IV) was added and the liver was perfused for a further 4 min with Hanks balanced salt solution. After gentle shaking, the suspension was filtered and hepatocytes were sedimented at 50 ×g for 3 min. Non-parenchymal cells from the treatment were collected and sedimented at 300 ×g for 10 min. The pellet of nonparenchymal cells was resuspended and cultured with RPMI 1640 medium (containing 15 mmol/L HEPES, 0.05 U/mL insulin, 15 mmol/L L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin) supplemented with 10% newborn calf serum. After 30 min, the non-adherent cells were deleted. The viability of the KC was greater than 90% as determined by trypan blue exclusion.
Kupffer cells were fixed on a plastic dish and the phalloidin-TRITC (Sigma) method was used for staining actin by a previously reported method[3]. The samples were observed under fluorescence microscope (Olympus VANOX) and the actin contents were determined using a fluorometer ( Hitachi MPF-4 ). Indirect immunochemical staining was performed for myosin and vimentin using anti-myosin antibody and anti-vimentin antibody(Sigma)[4]. The samples were observed by using a 40-fold objective (Olympus VANOX). The staining images were collected by means of a Panasonic CL320 videotape camera and sent into an image analyzing computer system (CMIAS007) for quantitative assessment of the gray scale.
The phagocytic function of Kupffer cell was quantitatively measured by a method previously reported[4,5]. Cultured Kupffer cells were incubated with RPMI 1640 medium supplemented with 10% fetal calf serum at 37 °C for 6 h. After the incubation, 1.7 × 108 polystyrene beads/dish (diameter 1.1 μm, Sigma) were added to the cultures, which were maintained for a further 60 min at 37 °C. The cultures were washed three times with RPMI 1640 medium and the numbers of beads in Kupffer cells were counted under an inverted phase contrast microscope (XSJ-D), Chongqing Optical Electric Appliances Plant). One hundred Kupffer cells randomly selected from each of 5 different cell preparations were used for the phagocytosis study.
The results were expressed as -x±s and statistical analyses were made with one-way analysis of variance and Student’s t test.
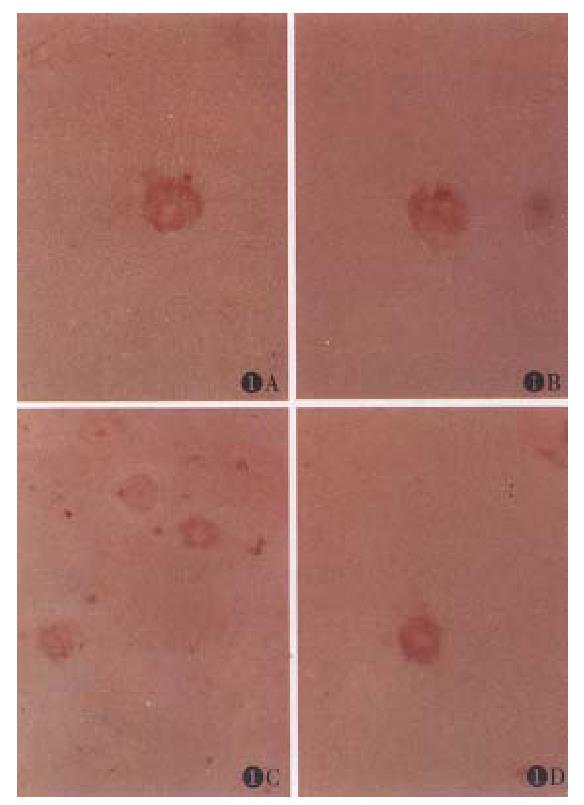
Aging caused morphological changes in cytoskeleton system of Kupffer cells are shown in Figure 1. Kupffer cells of 6-mo-old rat appeared flat and there were several pseudopodia on the surface. The myosin was shown in the pseudopodia. In fluorescent staining for actin, intense specific fluorescence was observed in both the cytoplasm and the peripheral region along pseudopodia. The distribution of actin was similar to that of myosin. Vimentin positive fibres extended throughout the whole cytoplasm and a perinuclear accumulation was also present in 6-month-old Kupffer cells. Kupffer cell of 24-month-old rat appeared small and circular and the number of pseudopodia decreased. Weak and diffuse fluorescence and staining could be observed around the pseudopodia and the nuclei of the aged cells in actin and myosin staining respectively. Weak staining could also be observed throughout the cytoplasm of the aged Kupffer cells in vimentin staining.
Aging resulted in significant reductions of the 3 cytoskeleton components mentioned above, i.e., the contents of actin, myosin and vimentin in 24-mo-old KC were significantly decreased to 68.0%, 84.9% and 75.5% respectively of those in 6-mo-old KC (P < 0.01,0.01 and 0.01), (Table 1).
In the 6-mo group, 19.6 ± 2.1 beads were taken up by cultured Kupffer cells during the 60 min observation period. In 12-mo, 18-mo and 24-mo groups, 20.8 ± 2.1, 12.7 ± 1.5, 8.6 ± 2.3 beads were taken up into Kupffer cells respectively. The difference between 6-month-old group and 12-mo-old group was not significant (P > 0.05). The difference between 6-mo-old group and 18-mo-old group and that between 6-mo-old group and 24-mo-old group were significant (P < 0.05).
Significant positive correlation was found between the changes of actin, myosin and vimentin contents in Kupffer cell aging and the damaged phagocytosis. γ values were 0.96 (P < 0.05), 0.99(P < 0.01) and 0.95(P < 0.05), respectively.
Kupffer cell is the main component of the host monocyte-macrophage system. It is crucially important for the host to fight against infection or sepsis[6]. The previous study showed that the decreased phagocytosis of the aged Kupffer cell was responsible for the increased severity of pathophysiological changes after endotoxemia[7]. So it is of significance to study the mechanism of decreased phagocytosis in Kupffer cell aging.
A 24-mo-old rat was used in the present experiment as the aging model, which was comparable to the age of 65-75 in the human being, an age period consistent with the standard of old people of our country. So 24-mo-old rat may serve as a qualified model for the study of Kupffer cell aging[2].
The mechanism of phagocytosis by Kupffer cells is still not completely understood. The ruffling of cell membrane and formation of pseudopodia play an important role in the phagocytosis of Kupffer cells and this is believed to be accomplished by the cytoskeleton. In the cytoskeleton, actin-myosin interaction through the calcium-calmodulin systems plays a major role in this activity[8]. In this system, intracellular Ca2+ combined with calmodulin to form the active calcium-calmodulin complex, which activates an enzyme, myosin light chain kinase, for phosphorylating the light chain of myosin. Phosphorylated myosin, but not unphosphorylated myosin, can interact with actin to induce the activity of cell membrane and pseudopodia and then phagocytosis. The process of this system is reversible, in that a phosphatase can catalyze dephosphorylation of myosin, restoring it to a form that can not be activated by actin.
Vimentin is another important cellular cytoskeleton component. It is in radial arrangement in cytoplasm, forming a frame to support the actin-myosin system and other organelles. It can prevent the cell from being injured by changing its tension, thus keeping the cellular shape.
In the current study, the distribution and determination of actin, myosin and vimentin in Kupffer cells at various ages were studied and our observations reve aled that the distributions of the 3 cytoskeletons dramatically changed and their contents significantly decreased in aged Kupffer cells. The actin fluorescence and myosin staining in the area of the pseudopodia became weak in the aging Kupffer cells. The vimentin staining throughout the whole cytoplasm became weak too. These results indicate that the age-related damage of the cytoskeleton system in Kupffer cell aging is one of the important mechanism responsible for a decre ase in phagocytosis.
Granted by the Military “8th Five” Research Funds.
| 1. | Brouwer A, Parker SG, Hendriks HF, Gibbons L, Horan MA. Production of eicosanoids and cytokines by Kupffer cells from young and old rats stimulated by endotoxin. Clin Sci (Lond). 1995;88:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Sun WB, Han BL, Peng ZM, Ma RL, Cheng J, Duan HC et al. Establishment and evaluation of Kupffer cell model for aging study. Chin J Geriat. 1995;14:357-359. |
| 3. | Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA. 1979;76:4498-4502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 556] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Hirose M, Watanabe S, Ueno T, Kitami N, Sato N. Pertussis toxin-induced redistribution of cortical actomyosin and inhibition of phagocytosis in rat Kupffer cells. J Gastroenterol Hepatol. 1993;8:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Sun WB, Han BL. Chen J, Ma RL, Duan HC, Wang HZ. The effect of aging on the Kupffer cell's phagocytosis and susceptibility to endotoxin. Chin J Expe Surg. 1995;12:263-264. |
| 6. | Albright JW, Albright JF. Ageing alters the competence of the immune system to control parasitic infection. Immunol Lett. 1994;40:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Knook DL, Brouwer A. Kupffer cells and the acute phase response: the effect of aging. Immunol Invest. 1989;18:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bretscher A. Microfilament structure and function in the cortical cytoskeleton. Annu Rev Cell Biol. 1991;7:337-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 211] [Article Influence: 6.2] [Reference Citation Analysis (0)] |