Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.74
Revised: June 19, 1997
Accepted: August 3, 1997
Published online: February 15, 1998
AIM: To explore a simplified method for isolation of hepatocytes and establish a method of primary hepatocyte culture with more aggregates and longer persisence.
METHODS: Wistar rat hepatocytes were isolated by a single extracorporeal two-step perfusion method, and the cells were seeded on poly-HEMA coated flasks and cultured with hormonally defined medium and gentle shaking at regular intervals.
RESULTS: The total yield of isolating hepatocytes amounted to 108 cells for each rat liver with the viability of more than 90% in all isolations. Under the nonadherent environments, the cells were found to attach to each other and form multicellular aggregates rapidly, and the aggregates became spheroidal shape after two days in culture. The morphologic characteristics and albumin synthetic function of the multicellular spheroidal aggregates can be maintained for one month.
CONCLUSION: The simple and reliable isolation as well as large scale and longer time culture of hepatocytes can be used for experiments in liver cell transplantation and bioartificial liver support system.
- Citation: Wang YJ, Li MD, Wang YM, Ding J, Nie QH. Simplified isolation and spheroidal aggregate culture of rat hepatocytes. World J Gastroenterol 1998; 4(1): 74-76
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/74.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.74
Hepatocyte culture, as an optimal model in vitro, plays an important role in the studies of liver disease[1]. However, the collagenase perfusion technique in situ can not be performed easily, the usual culture of hepatocytes, seeded on tissue culture dish under classical culture conditions can not adapt to large scale and high density culture and can not maintain liver-specific functions for longer time, thus limiting the development and application of hepatocytes culture. In this study, we used a simplified two-step perfusion method and some effective measures to isolate the rat hepatocytes in vitro and culture hepatocyte as spheroidal aggregates.
Wistar rats weighing 150 g-200 g, were provided by the Experimental Animal Centre of Third Military Medical College. The animals have received standard laboratory diet.
Liver cells were isolated by an adaptation of the two-step perfusion method[2]. Briefly, the animals were anesthetized with barbital (30 mg/kg, b. w, intraperitoneally) and their livers were removed intact. The liver was first perfused in vitro via the portal vein with warmed (37 °C) Ca2+ and Mg2+ free Hanks balanced salt solution at a flow rate of 5-8 mL/min for 10-15 mL/min, and then perfused with 0.05% collagenase (Sigma, Type IV) in the same solution supplemented with 5 mM CaCl2 and 50 mM HEPES. The reperfusion with collagenase soultion lasted 20 min at a rate of 5 mL/min at 37 °C. After 10min of incubation (37 °C) with gentle shaking, the suspension was filtered and hepatocytes were sedimented at 50 g for 3 min.
Freshly isolated liver cells (4 × 105 in 1 mL of RPMI 1640 medium) were seeded in culture flasks precoated with poly (2-hydroxyethyl methacrylate) (poly-HEMA, Sigma) and cultured in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. In order to inhibit cells attachment on wells and promote cells agregation, the culture flasks were rotated at a re-gular interval. After 4 h of incubation, RPMI 1640 medium was changed by a hormone defined medium (HDM) composed of Williams medium E supplemented with 10 mg/L insulin, 10 μg/L HGF, 50 μg/L EGF, 100 μg/L hydrocortisone, 100 μg/L glucagon, 50 mg/L linoleic acid, 100 U penicillin, 0.1 mg/mL streptomycin, 1 mg/L amphotercin B and 10% FCS (mainly reagents were purchased from Sigma). The medium was changed every day.
Cell morphology in the culture was observed under Olympus phase contrast microscope. For electron microscopy the spheroidal aggregates of hepatocytes cultured for different time periods were fixed at 4 °C for 4 h with 3% glutaraldehyde in 0.1 M phosphate buffer and post-fixed for 1 h with 1% OsO4, dehydrated in graded ethanol solutin and embedded in Epon 618. Ultrathin sections were prepared coventionally and observed with a JEM-2000SX transmission electron microscopy (TEM). Scanning electron microscopy (SEM) sample was dehydrated by the same method and further dehydrated by critical point-drying. A thin-layer of gold was deposited on the cell surface by a sputtering system and examined in AMRAY 1000B SEM.
The albumin synthesis by hepatocytes in culture media was determined on the Beckman CX-7 Synchron Clinical System.
The total yield of isolated hepatocytes by the simplified two-step perfusion method amounted to 2-4 × 108 cells for each liver. The viability of hepatocytes as judged by the trypan blue test was estimated as 90%-98% in all isolations.
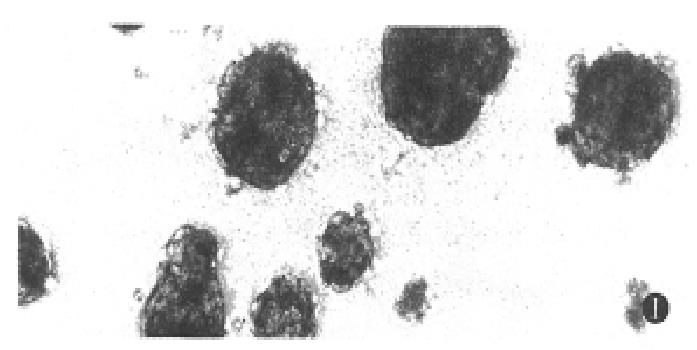
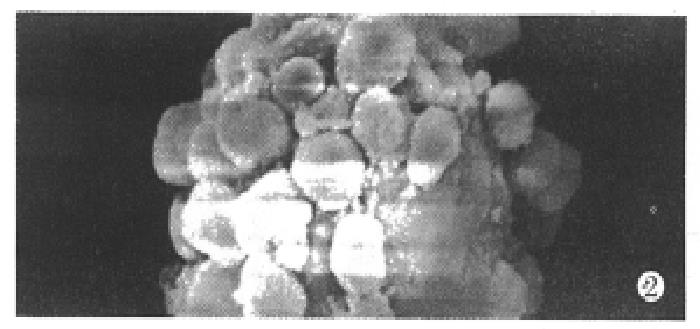
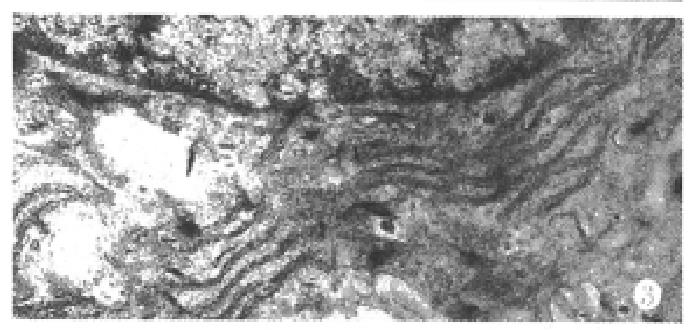
Under the defined culture environment and repeated gentle shaking, the freshly isolated hepatocytes attached to each other within 4 h-8 h and multiple aggregates of different sizes were loosely formed and the multiplicity of aggregates increased along with time. Up to 48 h, a lot of regular spheroidal aggregates were seen in flasks. The aggregate hepatocytes spheroids appeared very tight and dense in centre, and on the surface of the spheroids attached a lot of single cells as flower (Figure 1, Figure 2). Through action of single cells, some spheroids were also attached to each other. The characteristics of spheroidal aggregates can be maintained until the 30th day of culture. At the same time, TEM revealed that the cultured hepatocytes had a large round nucleus and abundant cytoplasmic organelles as observed in normal ones (Figure 3).
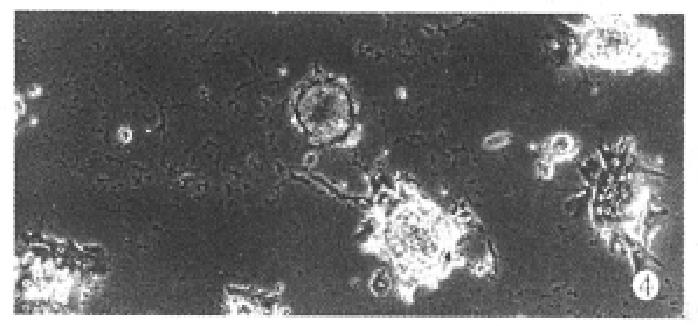
When the aggregate culture stopped, cell spheroids were transferred into collagen-coated wells. After 24 h of incubation (37 °C, 5% CO2) approximately, 74%-81% spheroids were seen to attach on substratum and many cells incorporated into spheroids migrated out forming a monolary (Figure 4).
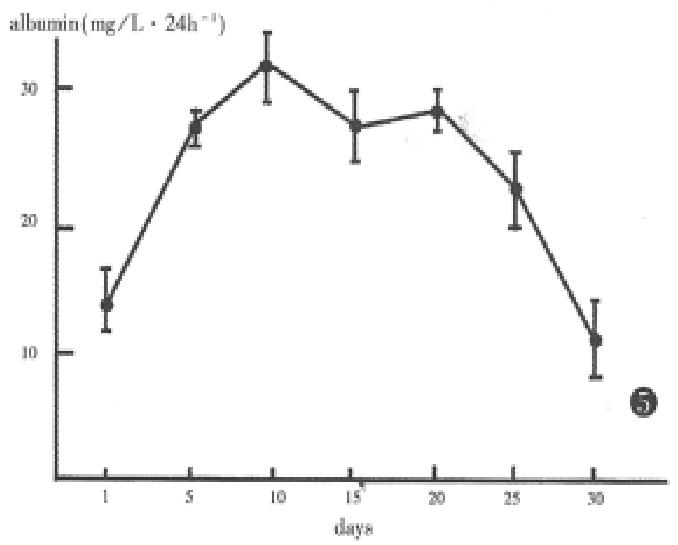
Secretion of albumin by hepatocytes was virtually detectable in the aggregate culture. By 5 days in culture, a marked increase in albumin production was seen, which can be maintained until the 25th day of culture. By 30 days, albumin secretion decreased rapidly (Figure 5).
Until recently, hepatocytes are isolated according to the two-step perfusion method devised by Seglen. But as the method required a special apparatus and a lot of collagenase for liver perfusion in situ it is not so easy to completely dissociate the hepatocytes and to perform hepatocytes culture in ordinary laboratories. In our experiment, the rat liver was removed and perfused with colla genase in vitro. Although the yield of isolated hepatocytes is relatively low, the number still amounted to 108 cells and more than 90% of hepatocytes were viable. The equipment necessary for use was simple, the dose of collagenase was much lower than by Seglen method, and it can be adjusted according to the requirements of hepatocytes. These denote that the collagenase perfusion in vitro is a simple and reliable method for isolating hepatocytes from the biopsied samples and animal livers, which may be useful in performing hepatocytes culture widely.
Under the standard monolayer culture conditions, maintenance of survival and different functions of cultured hepatocytes have proven to be difficult. It is also difficult to provide large scale production of hepatocytes for some special use. Therefore some attempts have been made to increase the functional longevity and large scale production of hepatocytes in primary culture. For example, cultures of rat hepatocytes on Biosilon microcarriers allow one to obtain large metabolic active cells[3], cocultures of human or rat hepatocytes with rat liver epithelial cells allow the maintenance of several liver functions up to 2 months[4].
But the microcarrier method and coculture are unfavourable in use. Since the culture of neonatal rat liver cells in spheroidal aggregates was described by Landry et al[5], special attention has been paid to the culture method. Because the spheroids were formed in three-dimensional structures where cell-cell contacts are maximized, and the reaggregate and re-form structures resembled in some aspects of those found in vivo. Under those cultural conditions, the hepatocytes can mainta in viability and functional integrity for months. Our experimental results were in agreement with that, albumin was still secreted by cell aggregates up to 30 days of culture, at the same time, the cells were still capable of attaching collagen-coated wells possibly due to the effective methods including poly-HEMA, HDM, cell growth factors and a rotating method.
Culturing of hepatocytes as spheroidal aggregates may be preferable to monolayer culture as an experimental liver model. The exciting potential applications may include the use of these hepatic spheroids for investigation in therapeutic measures for acute hepatic failure, such as hepatocytes transplantation and the construction of extracorporeal bioartificial liver support systems.
Project supported by the National Natural Science Foundation of China, No. 39370189.
| 1. | Moshage H, Yap SH. Primary cultures of human hepatocytes: a unique system for studies in toxicology, virology, parasitology and liver pathophysiology in man. J Hepatol. 1992;15:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Seglen PO. Preparation of rat liver cells. II. Effects of ions and chelators on tissue dispersion. Exp Cell Res. 1973;76:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 184] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Shnyra A, Bocharov A, Bochkova N, Spirov V. Large-scale production and cultivation of hepatocytes on Biosilon microcarriers. Artif Organs. 1990;14:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Clement B, Guguen-Guillouzo C, Campion JP, Glaise D, Bourel M, Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984;4:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 134] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 398] [Article Influence: 10.0] [Reference Citation Analysis (0)] |