Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.66
Revised: June 14, 1997
Accepted: July 30, 1997
Published online: February 15, 1998
AIM: To produce a new rat model of portal hypertension by intraportal injection of microspheres. METHODS: Measured aliquots of single or different-sized microspheres (15, 40, 80μm) were injected into the portal vein to block intrahepatic portal radicals. The resultant changes in arterial,portal,hepatic venous and splenic pulp pressures were monitored. The liver and lungs were excised for histological examination.
RESULTS: Portal venous pressure was elevated from basal value of 0.89-1.02 kPa to a steady-state of 1.98-3.19 kPa following the sequential injections of single- or different-sized microspheres, with a markedly lowered mean arterial pressure. However, a small-dose injection of 80 μm microspheres (1.8 × 105) produced a steady-state portal venous pressure of 2.53 × 0.17 kPa, and all rats showed normal arterial pressures. In addition, numerous microspheres were found in the lungs in all experimental groups.
CONCLUSION: Portal hypertension can be reproduced in rats by intraportal injection of microspheres at a small dose of 80 μm (1.8 × 105). Intrahepatic portal-systemic shunts probably exist in the normal rat liver.
- Citation: Li XN, Benjamin I, Alexander B. A new rat model of portal hypertension induced by intraportal injection of microspheres. World J Gastroenterol 1998; 4(1): 66-69
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/66.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.66
Portal hypertension is associated with gross haemodynamic disturbances in portal and systemic circulations. Animal models are still important for research into portal hypertension. One of the most popular models used is the partial portal venous ligation model in the rat[1], but this can only achieve extrahepatic portal venous occlusion and is not representative of intrahepatic portal hypertension as seen clinically. Carbon tetrachloride induced models of cirrhosis closely resemble the major features of the human disease[2], but take a long time to deve-lop and are associated with a high mortality and a wide heterogeneity in the stage and development of cirrhosis[3,4]. In the present study, a new rat model of portal hypertension was successfully induced by intraportal injection of microspheres.
Thirty-eight Sprague-Dawley rats weighing 250 g-350 g were randomly divided into six groups. Groups 1 (n = 6) and 2 (n = 6) received sequential injections of single-sized microspheres of 15 and 80 μm diameters respectively. Groups 3 (n = 6) and 4 (n = 6) were given sequential injections of different-sized microspheres in order of size 15, 40 and 80 μm and 80, 40 and 15 μm, respectively. According to the results of the four groups above, two bolus injections of 80 μm microspheres were selected as the suitable dose for the induction of portal hypertension in the model and these were given to Group 5 (n = 8) for further observation. Rats in Group 6 (n = 6) were injected with saline and served as controls.
The animals were anaesthetised with fentany/fluanisone (0.3 mL/kg, subcutaneously ) and midazolam (0.3 mL/kg, subcutaneously ). Mean arterial pressure (MAP) was monitored using a catheter in the left carotid artery. The abdomen was opened via a midline incision and the portal vein was cannulated through an ileocolic vein for measurement of portal venous pressure (PVP) and injection of microspheres. The splenic pulp pressure (SPP) was measured through a 23G butterfly scalp needle. Wedged hepatic venous pressure (WHVP) was measured in Groups 1, 2, 5 and 6. All of the cannulae were connected to P23XL (Viggo Spectramed Inc.) pressure transducers, and permanent recordings were made on a polygraph recorder (Grass Instruments Inc., USA). When steady basal pressures had been achieved for at least 5 min, injections of microspheres or saline started. Before injection, latex microspheres (Coulter Electronics Ltd., England) were agitated for 60 seconds. In each injection, microspheres were given iav the portal venous catheter in a volume of 0.2 mL and immediately followed by 0.2 mL saline injection to flush the catheter. Only when a steady PVP has been achieved for at least 5 min was the next injection given. In Group 5 there was no interval between the two injections. The numbers of microspheres used are shown in Table 1. Following completion of the injection, animals were observed for 10-30 min until the final steady PVP had been reached. Finally a vascular clamp was applied to the portal vein at the liver hilus and the pressures monitored. After this, the animals in Group 6 received partial portal venous ligation. At the end of the experiment, all rats were killed by opening the chest. The liver and lungs were taken and fixed in 10% formal-saline for histologicalexamination.
| Group | Sphere diameter (μm) | No of aliquots | No ofmspheres/aliquot | Total No of spheres |
| 1 | 15 | 6 | 5.6 × 106 | 3.4 × 107 |
| 2 | 80 | 5 | 9.0 × 104 | 4.5 × 105 |
| 3 | 15,40,80 | 15 μm× 2 | 5.6 × 106 | 1.1 × 107 |
| 40 μm× 2 | 2.4 × 105 | 4.8 × 105 | ||
| 80 μm× 2 | 9.0 × 104 | 1.8 × 105 | ||
| 4 | 80,40,15 | 80 μm× 2 | 9.0 × 104 | 1.8 × 105 |
| 40 μm× 2 | 2.4 × 105 | 4.8 × 105 | ||
| 15 μm× 2 | 5.6 × 106 | 1.1 × 107 | ||
| 5 | 80 | 2 | 9.0 × 104 | 1.8 × 105 |
Results were expressed as mean ± standard error. Comparisons were made by means of t test. Results were considered statistically significant at P < 0.05.
MAP decreased by approximately 45% following the first injection of microspheres in all experimental groups (kPa, 12.95 ± 1.66 vs 7.32 ± 0.68, P < 0.001). MAP in Group 5 eventually returned to normal levels after approximately 40 min following the two injections of 80 μm microspheres. No change in MAP was observed in the control rats during saline injections and after partial portal venous ligation. However, portal venous occlusion in control rats produced a significant reduction in MAP (kPa, 12.86 ± 1.65 vs 6.88 ± 1.14, P < 0.001), si-milar to that seen after microsphere injections in the experimental groups (P > 0.05).
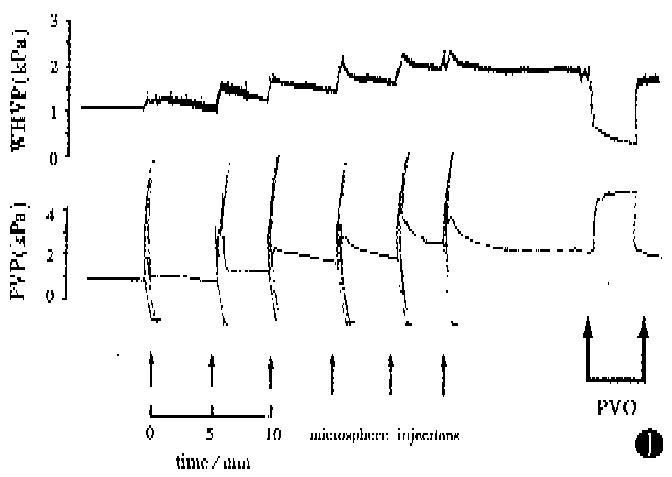
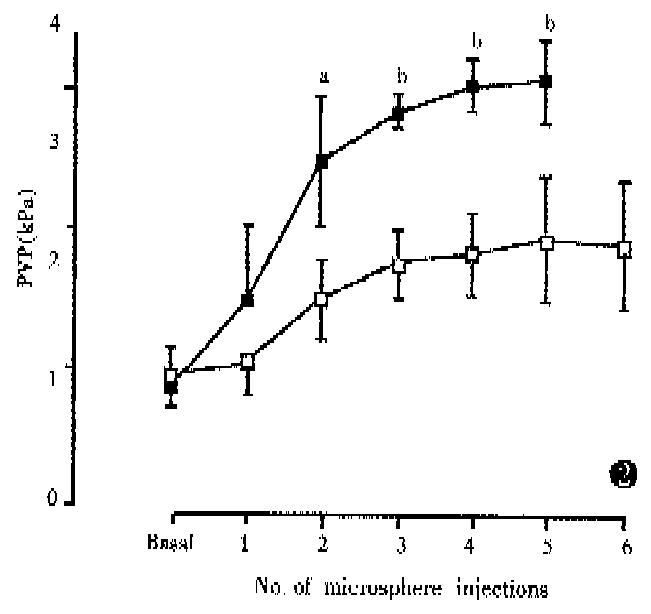
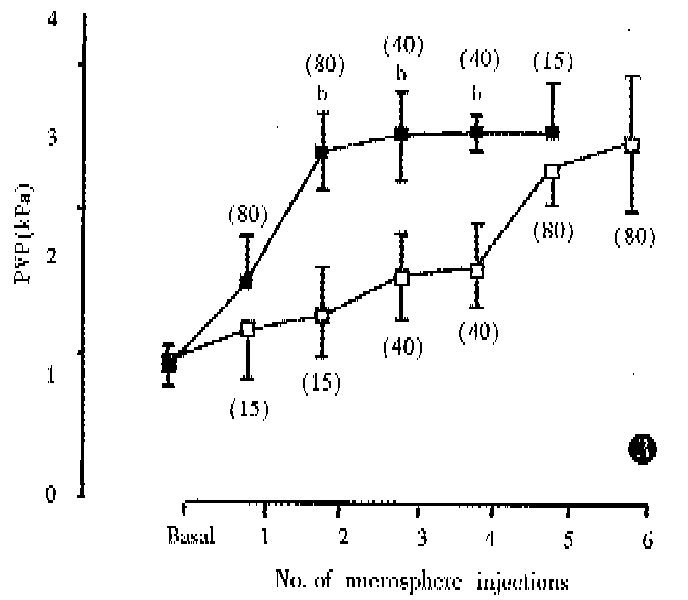
PVP rose gradually following the microsphere injections and, in Group 1, synchronous increase in WHVP was found (kPa, 0.93 ± 0.13 vs 1.65 ± 0.24, P < 0.01) (Figure 1). In Group 2, 80 μm diameter microspheres produced a large, rapid increase in PVP (Figure 2) with a significant reduction in WHVP (kPa, 0.89 ± 0.09 vs 0.47 ± 0.09, P < 0.01). In Groups 3 and 4, a marked increase in PVP was only observed after injection of 80 μm diameter spheres (Figure 3). Four rats in Group 4 died within 2-17 min after the 6th injection of microspheres. In Group 5, two sequential bolus injections of 80 μm microspheres elicited animmediate reduction in WHVP (kPa, 0.92 ± 0.13 vs 0.47 ± 0.09, P < 0.01) and an immediate increase in PVP to 2.53 kPa ± 0.17 kPa, which remained elevated during the observation period of 150 min. There were no significant changes in PVP in the control group after injection of an equivalent volume of saline aliquots. Results are compared in Table 2. Portal venous occlusion with a clamp produced a further large, rapid rise in PVP (Figure 1) to 5.16 kPa ± 0.76 kPa,4.87 kPa ± 0.79 kPa,4.95 kPa ± 0.99 kPa, 5.55 kPa ± 0.89 kPa and 5.75 kPa ± 0.32 kPa in groups 1,2,3,5 and the control respectively. There was no significant difference between the va-lues in these groups (P > 0.05). Partial portal venous ligation in control rats induced a significant increase in PVP to 2.75 kPa ± 0.07 kPa and was similar to that in Group 5 (P > 0.05).
| Group | PVP(kPa) | SPP(kPa) | ||||||
| Basal | Post-infusion | Basal-Post | % increase | Basal | Post-infusion | Basal-Post | % increase | |
| 1 | 0.98 ± 0.21 | 1.98 ± 0.48a | 1.00 ± 0.37a | 102.2 ± 35.6a | 1.17 ± 0.39 | 2.01 ± 0.51a | 0.84 ± 0.44a | 90.9 ± 41.8 |
| 2 | 0.89 ± 0.19 | 3.19 ± 0.29a | 2.30 ± 0.36a | 272.2 ± 78.0a | 1.44 ± 0.43 | 3.03 ± 0.33a | 1.60 ± 0.60a | 139.3 ± 51.7 |
| 3 | 1.02 ± 0.11 | 2.65 ± 0.49 | 1.64 ± 0.52 | 162.8 ± 60.1 | 1.24 ± 0.16 | 2.49 ± 0.45 | 1.24 ± 0.48 | 103.1 ± 48.5 |
| 4 | 0.98 ± 0.13 | 2.70 ± 0.13 | 1.72 ± 0.20 | 178.6 ± 41.0 | 1.16 ± 0.19 | 2.63 ± 0.13 | 1.49 ± 0.23 | 133.5 ± 39.2 |
| 5 | 1.04 ± 0.12 | 2.53 ± 0.17 | 1.45 ± 0.23c | 140.3 ± 32.0c | 1.44 ± 0.19 | 2.47 ± 0.19 | 1.04 ± 0.28c | 74.9 ± 26.6a |
| 6(control) | 1.04 ± 0.07 | 1.02 ± 0.05 | 1.36 ± 0.20 | 1.37 ± 0.19 | ||||
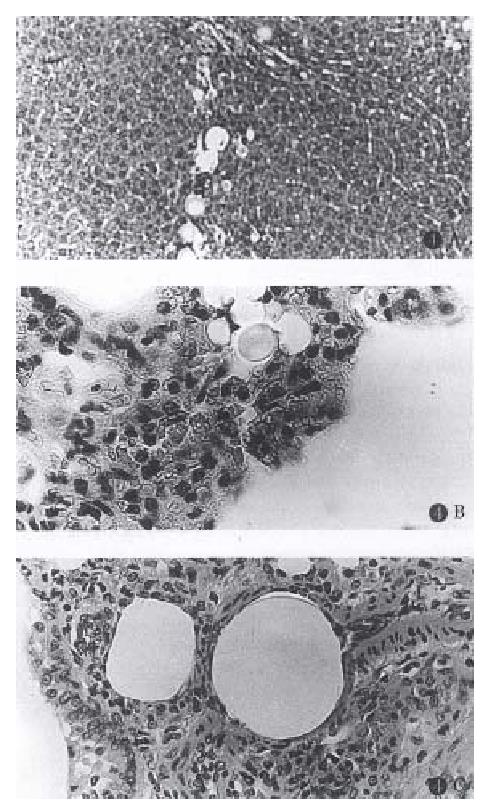
Histological examinations showed that in Groups 1 and 2 almost all of the portal radicles identified in the fields were blocked by the injected microspheres, with 15 μm spheres (Group 1) lodged in the terminal portal venules and 80 μm spheres (Group 2) lodged in the large portal radicles. In Group 3, the large and small portal radicles were simultaneously blocked by the different sized spheres injected. However, in Group 4, portal venules were mainly obstructed by the 80 μm spheres, while many 15 and 40 μm spheres injected after the 80 μm diameter spheres were trapped over the 80 μm spheres. The results in Group 5 were similar to Group 2, but with fewer spheres than those observed in Group 2. Numerous microspheres were found in the lungs of all experimental groups (Figure 4).
Anatomically the intrahepatic portal tract branches into progressively smaller radicles until the sinusoids are reached. Therefore, intraportal injection of microspheres can block the intrahepatic portal radicles and lead to presinusoidal portal hypertension. The present study confirmed that the PVP was elevated to a steady state 100%-270% above the basal value following microsphere injections. Obviously, the increase in PVP was related to the size and numbers of microspheres used: the 80 μm spheres caused a large, rapid rise in PVP compared to 15 μm spheres (Figure 2). As shown by the histology, the 80 μm spheres were trapped in large portal radicles and therefore smaller numbers would produce a marked PVP raising effect. The results of sequential injections of different-sized microspheres showed that the final PVP was dependent upon the effect of the largest spheres (Figure 3).
When intrahepatic portal radicles were blocked by the microspheres injected, the presinusoidal increase in resistance produced not only an elevated PVP, but also a markedly lowered MAP because of extensive mesenteric pooling of portalve nous blood. Therefore, both the PVP and MAP should be considered into the reproduction of this model. From the results of the first four groups, it was found that two injections (1.8 × 105) of 80 μm spheres created an augmentation in PVP with the recovery of MAP. Furthermore, all rats in Group 5 showed a normal MAP and high PVP of 2.53 kPa ± 0.17 kPa which was comparable to the value of 2.75 kPa ± 0.07 kPa in the control group after partial portal venous ligation, and to the value of 1.8 kPa ± 0.2 kPa found in our cirrhotic model previously induced by carbon tetrachloride[2]. These suggest that 1.8 × 105 of 80 μm spheres is the suitable dose for induction of portal hypertension in the model.
Clinically, portal hypertension is mainly caused by different forms of chronic liver disease, and is characterized by an increased resistance in the liver and by the formation of portosystemic collaterals. Though in early studies the site of the increased resistance was thought to be postsinusoidal, recent works have proved that the presinusoidal resistance is also increased in cirrhotic liver and contributes to the development of portal hypertension[5,6]. In particular, the presinusoidal block is the main cause of the idiopathic portal hypertension and the portal hypertension resulted from chronic biliary obstruction [7,8]. Therefore, this model, in regard to the location of increased intrahepatic resistance, possesses the general features of clinical cases. Moreover, the fact that many microspheres appeared in the pulmonary vascular bed suggested the opening of portosystemic collaterals during the procedure, with a diameter of more than 15 μm-80 μm.
Originally we believed that the presence of microspheres in the lung is only due to the opened extrahepatic collaterals, and this seems to be supported by the observation that the SPP was slightly lower than PVP. However, in this study although the PVP in groups 1 and 2 did not increase significantly after the 3rd injection, which implied that the intrahepatic portal radicles had been saturated by the microspheres injected, the final PVP achieved was substantially less than that obtained by portal vein occlusion. Consideration of the simultane ous changes of PVP and WHVP in Group 1 strongly suggested the existence of intrahepatic portal-systemic shunts in the normal rat liver. Opening of these shunts would permit portal blood flowing directly into the hepatic veins, leading to an elevation of WHVP and preventing further increase in PVP. When the portalvein occlusion was performed extrahepatically, the function of intrahepatic shunts was deprived, and, as a result, PVP could rise to an extremely high level. The intrahepatic shunts have not been described in the normal liver, but have been reported in cirrhotic livers in rats[9] and in humans[10]. It has been suggested that the frequency of large shunts (diameter > 25 μm) is relatively low and this is probably responsible for the reduction in WHVP after 80 μm microsphere injections in this study.
This model of portal hypertension is intrahepa-tic and can be induced rapidly, with the opening of intra- and extra-hepatic portal-systemic shunts. A major advantage of this model is that while the intrahepatic presinusoidal block is achieved acutely, the normal liver architecture remains. This may be particularly beneficial to the research in the actions of mechanical obstruction or some humoral substances, related to liver dysfunction[2], in the pathogenesis of portal hypertension. In the experimental cirrhotic model, it is difficult to differentiate these two actions.
Project supported partially by the King’s College Medical Research Trust and the Central Research Fund of the University of London.
| 1. | Geraghty JG, Angerson WJ, Carter DC. Portal venous pressure and portasystemic shunting in experimental portal hypertension. Am J Physiol. 1989;257:G52-G57. [PubMed] |
| 2. | Li XN, Huang CT, Wang XH, Leng XS, Du RY, Chen YF, Hou X. Changes of blood humoral substances in experimental cirrhosis and their effects on portal hemodynamics. Chin Med J (Engl). 1990;103:970-977. [PubMed] |
| 3. | Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis. Hepatology. 1983;3:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 198] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Sieber CC, Lopez-Talavera JC, Groszmann RJ. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology. 1993;104:1750-1754. [PubMed] |
| 5. | Shibayama Y, Nakata K. Localization of increased hepatic vascular resistance in liver cirrhosis. Hepatology. 1985;5:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Shibayama Y. On the pathogenesis of portal hypertension in cirrhosis of the liver. Liver. 1988;8:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Fukuda Y. Pathological study on Banti's syndrome. Acta Pathol Jpn. 1968;18:457-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Nayak NC, Ramalingaswami V. Obliterative portal venopathy of the liver. Associated with so-called idiopathic portal hypertension or tropical splenomegaly. Arch Pathol. 1969;87:359-369. [PubMed] |
| 9. | Wood AJ, Villeneuve JP, Branch RA, Rogers LW, Shand DG. Intact hepatocyte theory of impaired drug metabolism in experimental cirrhosis in the rat. Gastroenterology. 1979;76:1358-1362. [PubMed] |
| 10. | Hoefs JC, Reynolds TB, Pare P, Sakimura I. A new method for the measurement of intrahepatic shunts. J Lab Clin Med. 1984;103:446-461. [PubMed] |